Cevec is joining forces with US-based Paragon. The objective is to make Cevec’s scalable CAP expression technology the industry standard for glycoproteins and viral vectors in gene therapies.
![]() Based in Cologne (Germany), Cevec tackles with its human cell-based expression systems (CAP-GT and CAP-Go) two big challenges in Biotech manufacturing – industrial scale production of viral vectors and complex glycoproteins.
Based in Cologne (Germany), Cevec tackles with its human cell-based expression systems (CAP-GT and CAP-Go) two big challenges in Biotech manufacturing – industrial scale production of viral vectors and complex glycoproteins.
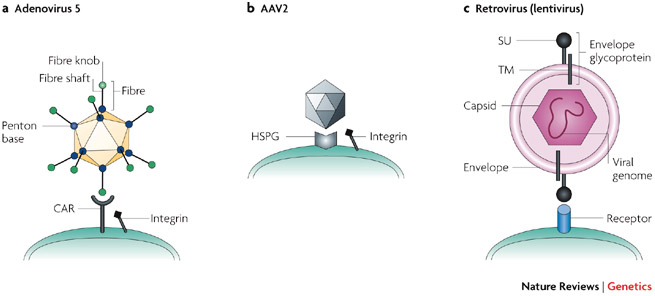
CAP-GT is a platform to produce viral vectors, such as lentivirus (LV), adenovirus (AV) and adeno-associated virus (AAV). These viral vectors are used to carry genetic information to the target cells in the patient’s body in gene therapy.
Cevec’s production of viral vectors has two key advantages – scalability and safety. Still, one of the biggest bottlenecks in gene therapy is the ability to produce reliable and safe vectors on an industrial scale to allow the commercial production of gene therapy products.
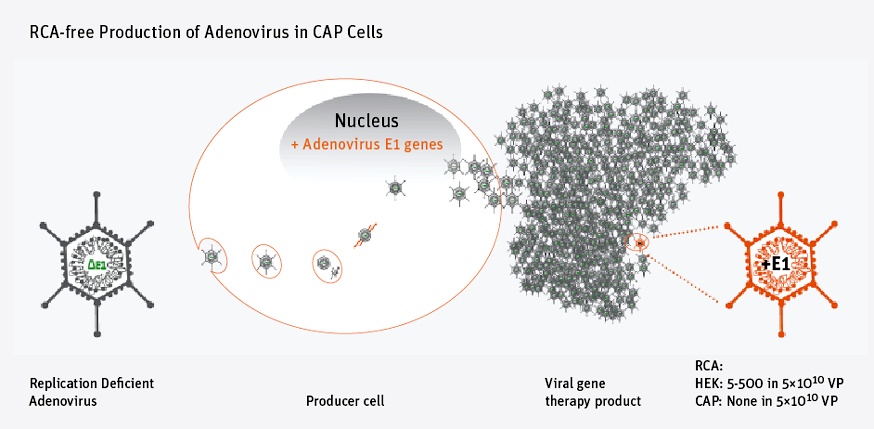
CAP–GT addresses both: it provides a fully scalable production process based on human suspension cell lines that proved to produce non-replicable adenoviruses. Unlike commonly used producer cell line HEK293 where replication-competent adenoviruses may occur given an inherent risk of homologous recombination of the virus DNA, CAP cells do not allow this kind of genetic recombination.
Cevec has now struck a deal with Paragon Bioservices (Baltimore, US), a contract development and manufacturing organization (CDMO) specialized in biopharmaceuticals and vaccines to roll-out its CAP technologies into the North American markets.
Paragon will become the preferred partner in North America, which Cevec will recommend to Biotechs using its CAP–GT system. Paragon will provide CDM services based on Cevec’s CAP technologies to its customers. Paragon will also be part of Cevec’s partners’ network in the rest of the world (including Europe). In this deal, Cevec will bring its expertise in evaluation and development of selected CAP cell lines, i.e. the generation of viral packaging and producer cell lines, along with requested licenses for Paragon customers using the CAP technology.
The two companies will also work together to develop and market a fully scaled-up production process for adeno-associated viruses (AAV), with capability to supply preclinical studies as well as Phase I and Phase II clinical trials.
This deal with Paragon could be the ideal partnership to support Cevec’s objective of making CAP-GT the standard technology for viral gene vectors. The timing may be just right, given the resurgence of gene therapies.
After years of regulatory caution, the first gene therapies (such as uniQure’s Glybera and GSK’s Strimvelis) are now arriving on the market – and there are many Biotechs working on others. These include Spark Therapeutics (tackling eye diseases) and the newly-founded Orchard Therapeutics, which will developing therapies for blood disorders.
Cevec is also rolling out its other platform CAP–Go in North America, an expression system for complex glycoproteins
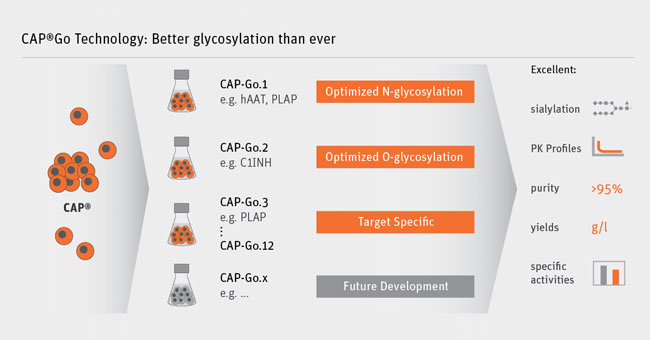
Producing recombinant proteins is the foundation of modern Biotech, with examples like Genentech’s biosynthetic insulin or Amgen’s Erythropoetin. However, some human proteins have complex modifications, such as added sugar molecules (glycolysation) – which are difficult to replicate in the manufacturing.
Relevant therapeutic biologics that can now be produced with Cevec’s technology include, besides complexly glycosylated proteins like coagulation factors and cytokines, alkaline phosphatases, which are being investigated as therapy for inflammatory and neurodegenerative diseases. A CAP-derived alkaline phosphatase and additional plasma proteins such as the difficult to produce human C1 Esterase-Inhibitor could be produced for the first time with pharmacokinetics matching or even exceeding the half-life of the serum purified molecule.
With its scale-up potential and strong manufacturing partners, Cevec could have a big role in the coming wave of new gene therapies.
Cevec’s CEO explains its future strategy for viral vectors…
Feature Credit Image: Virus © Cesar M. Romero (Dreamstime ID51265391)
Figure 1 Credit: Waehler et al. (2007) Engineering targeted viral vectors for gene therapy. Nature Reviews Genetics (doi: 10.1038/nrg2141)