Amgen is now months away from a European approval with Imlygic (also known as T-VEC) – the first oncolytic immunotherapy for advanced melanoma. The Committee for Medicinal Products for Human Use (CHMP) and the scientific committee of the European Medicines Agency (EMA), have reacted positively.
![]() Melanoma is a type of skin cancer that is characterized by the uncontrolled growth of melanocytes, which are the cells responsible for providing the pigment to skin. It is the most aggressive and serious form of skin cancer, and remains a significant public health concern in the EU. In 2012, it was estimated that there were 56,000 new cases of melanoma in France, Italy, Spain, Germany and the UK, causing almost 9,500 deaths.
Melanoma is a type of skin cancer that is characterized by the uncontrolled growth of melanocytes, which are the cells responsible for providing the pigment to skin. It is the most aggressive and serious form of skin cancer, and remains a significant public health concern in the EU. In 2012, it was estimated that there were 56,000 new cases of melanoma in France, Italy, Spain, Germany and the UK, causing almost 9,500 deaths.
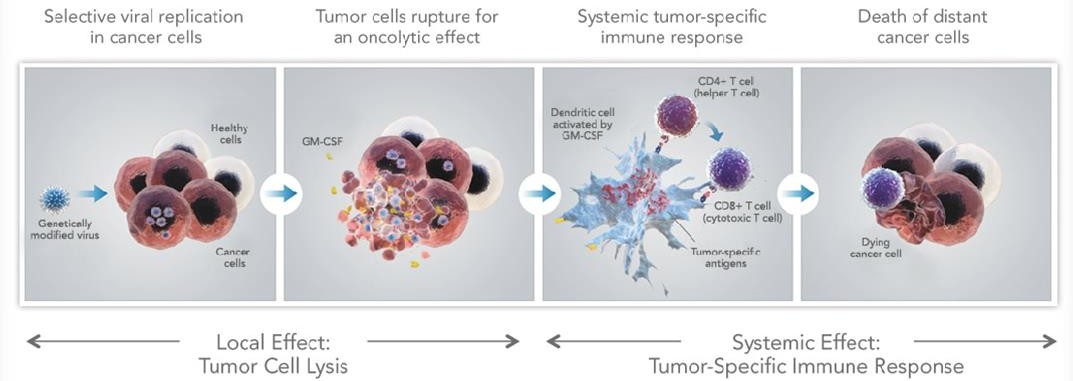
Amgen’s oncolytic immunotherapy is using a genetically modified strain of the herpes virus to invade tumors and replicate itself (e.g. OncoVex). Also known as talimogene laherparepvec (T-VEC) the viral-vectored kills cancer cells along the way and stimulates an immune response (by releasing GM-CSF tumoric antigens). This ‘reveals’ the cancer cells, further instigates the immune response and reduces risk of future relapse.
The positive opinion comes from a phase III trial evaluating the efficacy and safety of Imlygic in patients with Stage IIIB, IIIC or IV melanoma. It will serve as the basis for the European Commission’s decision on an EU-wide marketing authorization. Indeed, Amgen still needs to negotiate pricing with each member state.
Amgen focuses a lot on immuno-oncology partnerships like with Merck for Keytruda (pembrolizumab), as well as a deal with Roche to investigate the combination of Imlygic and the Swiss company’s investigational anti-PDL1 therapy (atezolizumab).
So, with Amgen’s T-VEC finally up to market access approval, the way for Oncolytic viral therapies is a novel path for the biotech Immuno-oncology industry to explore.