Roche has received market approval for atezolizumab to treat a common type of bladder cancer. It’s the 3rd checkpoint inhibitor on the market, and the first to target PD-L1.
 Immuno-oncology is a hype field that promises to revolutionize cancer treatment, with a lot of active research and big deals (like the €600M deal between argenx and AbbVie).
Immuno-oncology is a hype field that promises to revolutionize cancer treatment, with a lot of active research and big deals (like the €600M deal between argenx and AbbVie).
Checkpoint blocking is an important part of successful immuno-oncology strategies. This strategy uses antibodies to block the natural ‘safety checks’ of the immune system, stopping exaggerated responses – which can be quite… complicated.
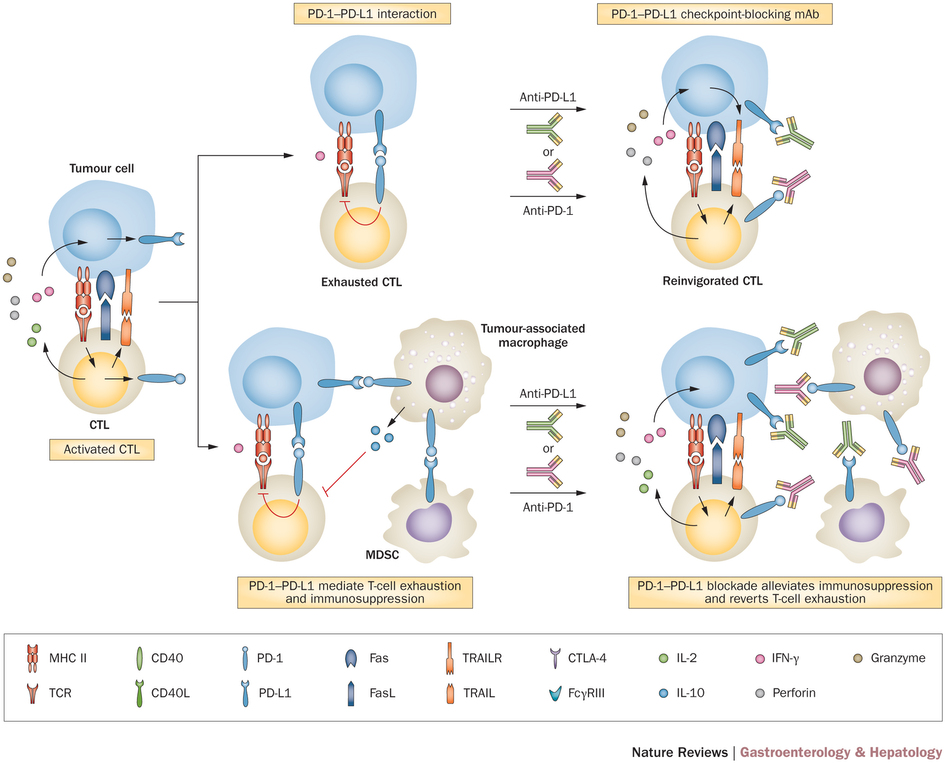
Checkpoint inhibitors are useful to stop cancer from hijacking this safety system to escape both natural immune response and immunotherapies. But until now, only two companies had succeeded in bringing products to the market in this area – MSD with Keytruda and Bristol-Myers Squibb with Nivolumab (but these were not PD-L1).
Now, Roche joins this exclusive group. It has just received FDA market approval for Tecentriq (atezolizumab), a checkpoint inhibitor that can now be used to treat a common type of bladder cancer – urothelial carcinoma.
Atezolizumab was in FDA’s Accelerated Approval Program, and the positive decision was made after data from a Phase II trial (IMVigor 210).
The candidate has shown to cause a tumor response in 26% (80 out of 310) of the patients with an adequate profile (PD-L1 positive).
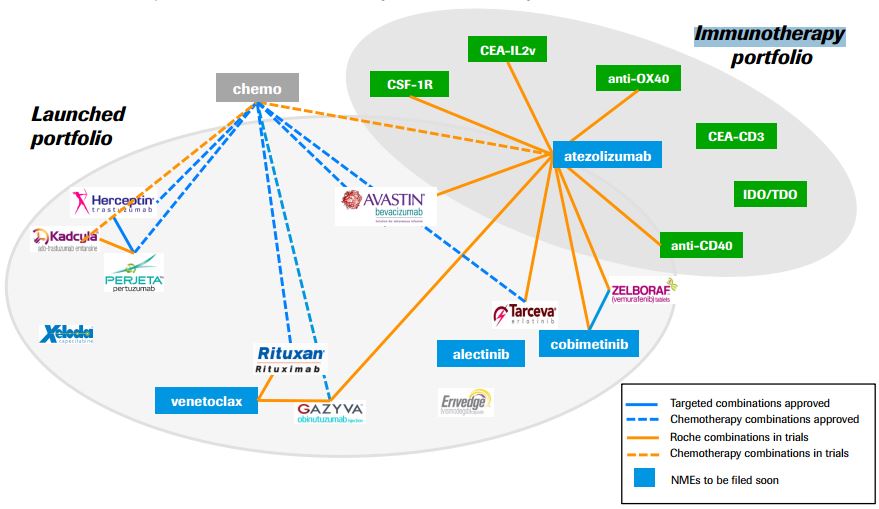
Besides being one of the few checkpoint inhibitors, atezolizumab is also the first of its kind. It targets PD-L1, a complementary pathway involved in cancers’ immune escape and a hype target in immuno-oncology.
The FDA also approved a complementary biomarker assay (Ventana PD-L1) to detect PD-L1 protein expression levels – another important part of immuno-oncology. The assay can assist doctors in determining which patients may benefit most from Roche’s treatment.
This approval is quite a win for Roche, which has focused in immuno-oncology to maintain its leading position in cancer therapies.