Nicox is filing a New Drug Application for its allergic conjuntivitis candidate, a novel eye drop formulation of a well-known antihistamine.
![]() Based in Sophia Antipolis (near the French Riviera), Nicox is developing therapies in ophthalmology.
Based in Sophia Antipolis (near the French Riviera), Nicox is developing therapies in ophthalmology.
Now, it has submitted one of its candidates (AC-170) for FDA approval, after good results from two phase III trials evaluating both the safety and efficacy in patients with allergic conjunctivitis.
Allergic conjunctivitis is an inflammation of the eye and inner surface of the eyelids – causing redness and itchiness. The indication has an annual market worth over €700M, with 75 million patients in the US.
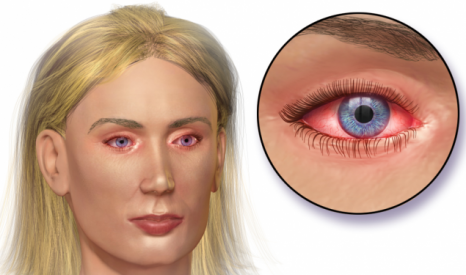
The disease is also quite common in children, and the pediatric results for AC-170 motivated Nicox to request a Priority Review. If this is granted, the candidate could be approved by end of 2016.
AC-170 is a novel eye-drop formulation of cetirizine, a compound already approved as an oral drug and commercialized as Zyrtec (by GSK). Cetirizine is a second generation antihistamine – a drug for allergies with fewer side effects in the nervous system, such as sedation.
It acts as a stabilizer for mast cells by binding to the histamine H1 receptor, which has an important role in inflammation.
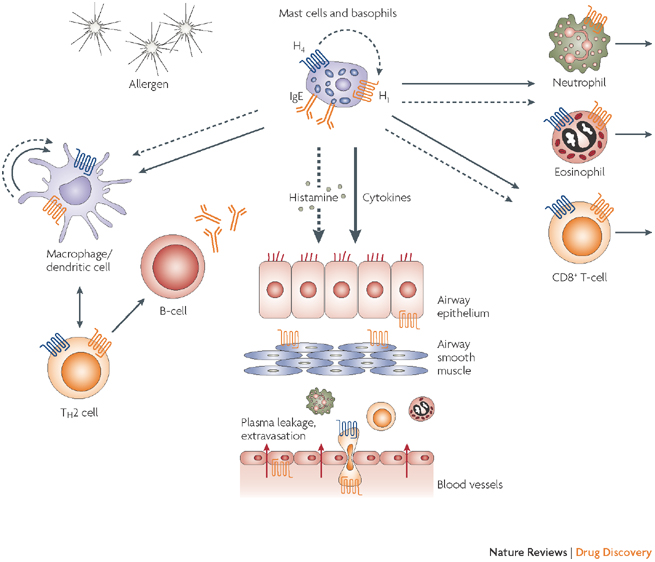
The candidate was initially developed by Aciex (US), which was acquired by Nicox in October 2014. Depending on approval, ex-shareholders could be looking at a milestone payment of over €30M ($35M).
Besides its acquisition strategy, Nicox also relies on nitric oxide-donating research platform. Nitric oxide (NO) is an important cell-signaling molecule, and a deficiency in the compound is involved in some eye disorders.
One of Nicox’ NO-donors, latanoprostene bunod (licensed to German Bausch + Lomb), also has a new drug application (NDA) under review by the FDA.
So Nicox could have two drugs approved during 2016 – not a small feat.
Figure 1 credit: Blausen.com staff (2014) Blausen gallery. Wikiversity Journal of Medicine (doi: 10.15347/wjm/2014.010)
Figure 2 credit: Thurmond et. al (2008). The role of histamine H1 and H4 receptors in allergic inflammation: the search for new antihistamines. Nature Reviews Drug Discovery (doi: 10.1038/nrd2465)





