Genfit reinforced its leading place in the race to find a cure for NASH when it announced this morning the launch of a program to validate their new diagnostic tool.
 Genfit is already leading in the development of a cure for Non-Alcoholic Stato-Hepatitis (NASH) with its candidate, Elafibranor, which is in Phase III trials. Now, the French €600M biopharma has announced that they will be testing the performance of their non-invasive diagnostic system in collaboration with the Antwerp Hospital in Belgium. They plan to expand this program across Europe and the US in the near future to reach thousands of patients.
Genfit is already leading in the development of a cure for Non-Alcoholic Stato-Hepatitis (NASH) with its candidate, Elafibranor, which is in Phase III trials. Now, the French €600M biopharma has announced that they will be testing the performance of their non-invasive diagnostic system in collaboration with the Antwerp Hospital in Belgium. They plan to expand this program across Europe and the US in the near future to reach thousands of patients.
NASH is a disease that affects 30 million people worldwide. The number of cases is increasing at an alarming rate in tandem with obesity and diabetes. However, there are currently no treatments available. As a result, many companies around the world are racing to be the first ones to reach the NASH market.
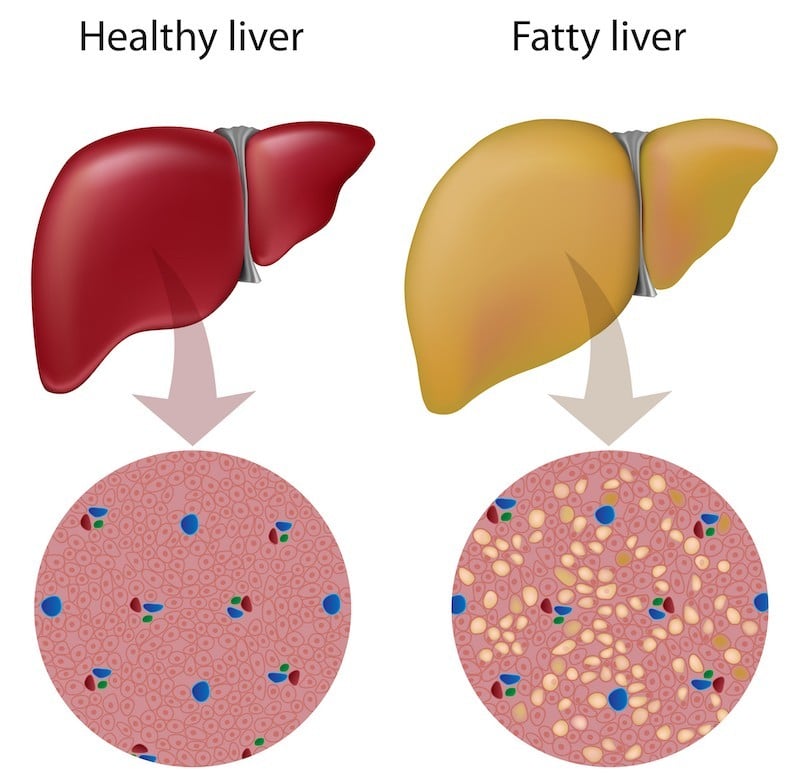
Right now, the diagnosis of NASH requires invasive histology to evaluate the liver. The new method announced by Genfit relies on the detection of miRNAs in the blood from patients. The company employs an algorithm to score the progression of the disease by taking into account the amounts of multiple miRNA biomarkers.
This tool will also facilitate the selection of patients that respond to Genfit’s Elafibranor, an oral drug for NASH currently in phase III trials. With this new announcement, Genfit establishes itself once again at the forefront of pharmaceutic companies tackling NASH.
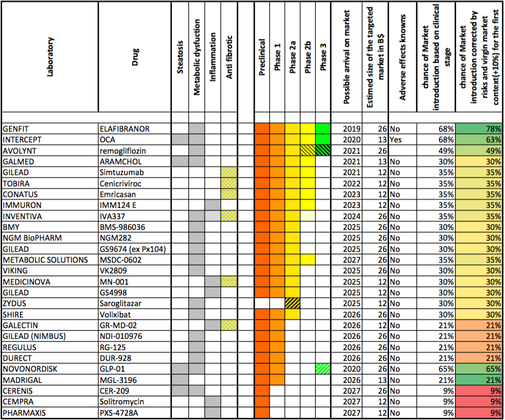
We interviewed Genfit’s CEO last year if you’d like to hear more about the company. It is head to head with US-based Intercept, the only other company worldwide which is in Phase III clinical trials. Who do you think will win the race? We’ll keep you updated.
Feature image credit: Etoileark/shutterstock.com
Figure 1 credit: Alila Medical Media/shutterstock.com
Figure 2 credit: nashbiotechs.com
Update 21/09: Genfit and Intercept are the only companies with a Phase III in NASH. It’s been updated in the article.