Agendia’s shows remarkable clinical results for its genomic assay (MammaPrint) allowed 48% of early-stage breast cancer patients to be spared from chemotherapy.
 Agendia started out in 2003 as a spin-off from the Netherlands Cancer Institute to commercialize diagnostic tools for cancer.
Agendia started out in 2003 as a spin-off from the Netherlands Cancer Institute to commercialize diagnostic tools for cancer.
Its first product was MammaPrint, which uses DNA microarray technology to test the activity 70 genes that affect breast cancer progression – and so the risk of recurrence or metastasis in the future.
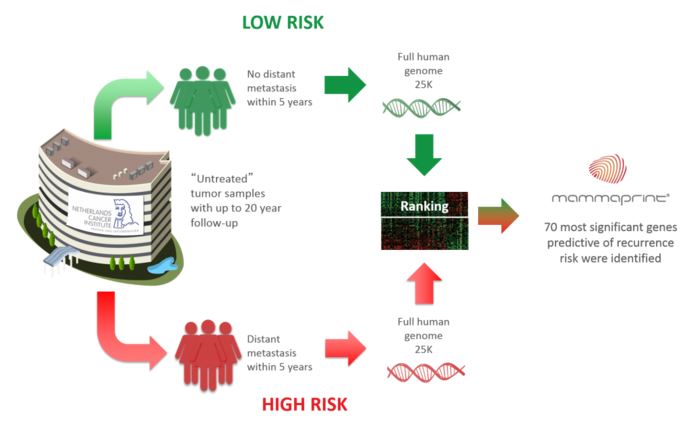
Traditional criteria of recurrence risk include age at diagnosis, size and type of tumor. After surgically removing the tumor, high risk patients are prescribed treatments like chemotherapy – which are both costly to health systems and aggressive for the patients.
MammaPrint can more accurately predict the risk of recurrence, including for a part of patients that would be high risk by clinical standards. So these individuals are spared from undergoing unnecessary chemotherapy – something worthy of the European inventor award.
Now, Agendia has presented important results at the recent AACR meeting (along with some other reputable European Biotechs).
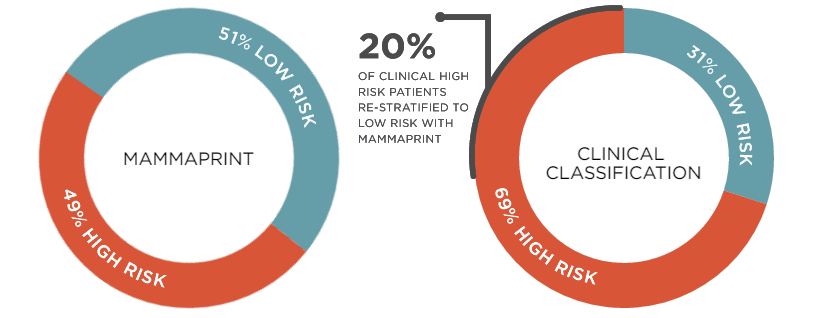
Its clinical trial (MINDACT) has shown a 46% overall reduction in chemotherapy – among high risk patients by clinical standards, calculated with the Adjuvant! Online tool.
Of the patients that were diagnosed as low risk by MammaPrint, 94% (3154 out of 3356) didn’t develop metastasis in the 5 years they were followed.
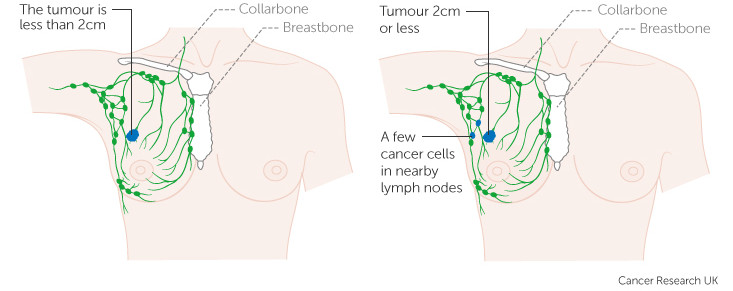
Notably, 48% (1610 out of 3356) of these patients were positive for lymph node involvement. Finding cancer cells in these ‘filters’ of the lymphatic circulation is usually deemed a sign of high risk – so the patient is prescribed chemotherapy.
MammaPrint has been available in Europe since 2004, and the FDA approved its initial 510(k) application (for medical devices) in 2007.
However, this trial is important to get further assurance for MammaPrint – which has now achieved the highest level of clinical evidence (1A). It also strengthens its position as the most robust test on the market, says Agendia’s CEO Mark Straley.
This is a big achievement for Agendia, which is definitely a success story in smaller companies in European Biotech.





