As presented last week, Genticel was getting back on track with the announcement of two major milestones with the aim to prepare the phase III. However, the major outcomes of its Phase II were expected to be presented on the 17th of June at Eurogin 2016 Congress… Here they are (and they are really bad)!
![]() GTL001 is designed to target and clear strain 16 & 18 of the Human Papillomavirus (HPV) from an infected body and avoid the development of cervical cancer, theoretically… Unfortunately, it seems like the treatment has some lack of activity. Indeed, the vaccine did not show any significant difference compared to the placebo.
GTL001 is designed to target and clear strain 16 & 18 of the Human Papillomavirus (HPV) from an infected body and avoid the development of cervical cancer, theoretically… Unfortunately, it seems like the treatment has some lack of activity. Indeed, the vaccine did not show any significant difference compared to the placebo.
The Phase II trial enrolled 233 patients – 117 have received two injections of 600 µg vaccines within 6 weeks and 116 the placebo with the same posology. After 18 months results showed that the clearance (elimination of the virus from the body) wasn’t higher in the first group than in the second one.
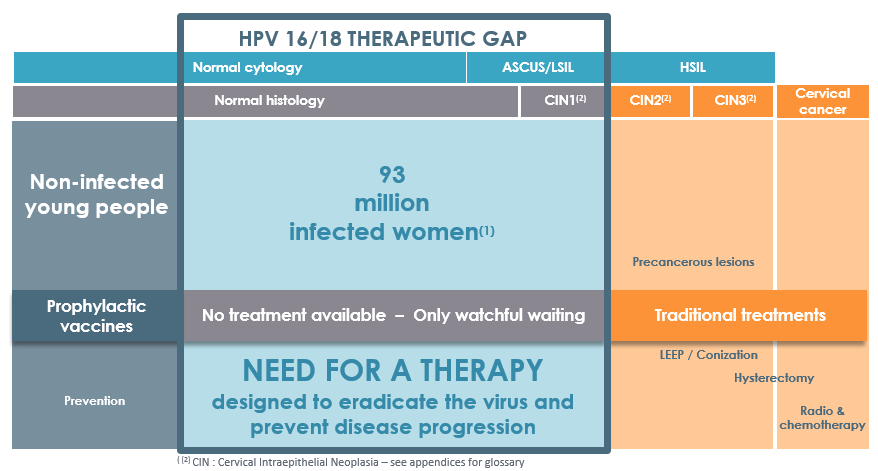
Genticel remains optimistic and is still assessing the development plan of the vaccine. The major reason is that the results of avoiding the potential development of cancer will be available in early 2017, after 24 months of assessment. It seems Genticel is not willing to stop the development of its lead candidate, but in regards to this bad announcement, it might be an option to save some cash.
Besides this, the company still has other products in development and is moving forward the deal with Serum Institute of India about Vaxiclase (the proprietary platform of Genticel). As a matter of fact, Benedikt Timmerman, the CEO of Genticel, has announced that those intermediary results do not affect the major development strategy of the company (e.g., other products and this deal).
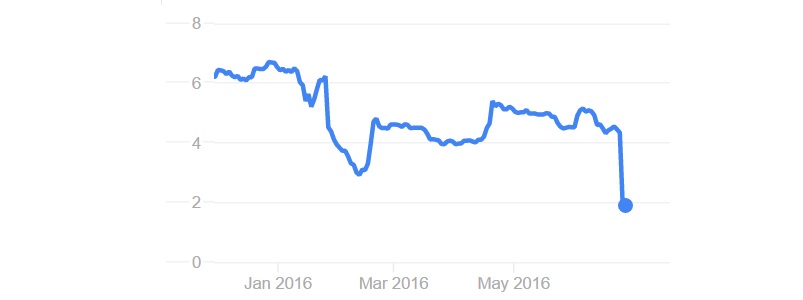
It doesn’t seem to have convinced investors. If you add the Brexit effect, you will get this kind of financial graph: the company’s stock dropped by over 70% after the announcement. The stock price is now 1.85€ compared to its initial listing price at over 7€. Its market cap is now only €30M, certainly far below what it raised so far.
Hard times for Genticel…
Feature Image Credit: Avalanche in Zinal (CC 3.0 Zacharie Grossen)





