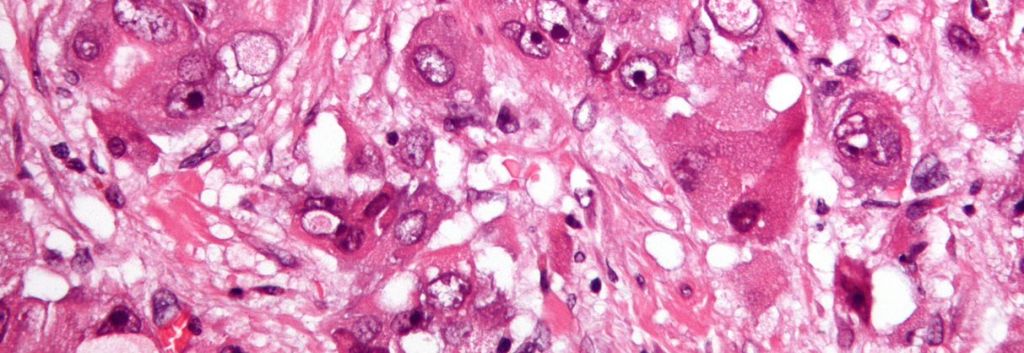
4SC, in Germany, has the FDA’s green light to conduct efficacy trials in the US with a candidate drug against hepatocellular carcinoma.
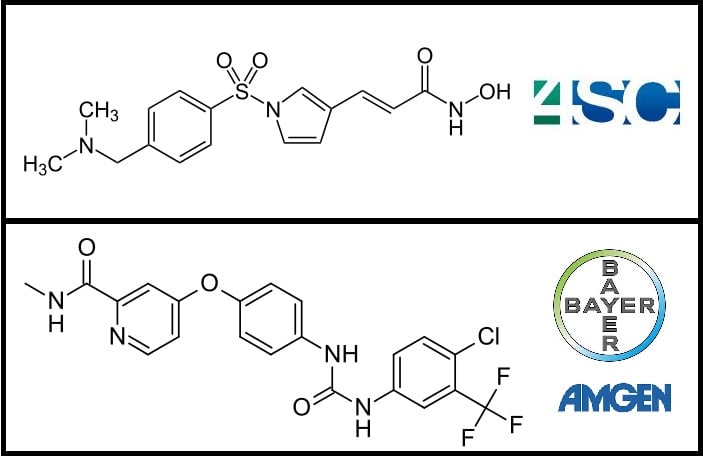
Munich-based 4SC’s lead candidate, resminostat, is a small-molecule being tested in several trials for some of the deadliest cancers. These include liver – which is also known as hepatocellular carcinoma (HCC).
Resminostat is an inhibitor of histone deacetylase (HDAC), that acts on an epigenetic level (an important part in cancer progression) to reprogram the behavior of cancer cells.
Advancing the trials for HCC in the US is an important milestone in the path towards worldwide commercialization, and will compare the efficacy of resminostat alone and in combination with sorafenib (nexavar).
Sorafenib is currently the only drug for treating HCC. Developed by German Bayer and Onyx Pharma (a subsidiary of US’s Amgen), this drug sparked controversy. The UK’s NHS blocked its use due to its high price, around €3,500 per patient per month, and relatively low efficacy, only an average of 6 months of added life expectancy for patients.
While there is a serious need a therapy to treat HCC, questions remain…
If this new therapy will just add a few more months of life expectancy, will it be enough to convince health systems and insurance companies of the benefit?
Well, when resminostat for HCC was combined with sorafenib in a phase II trial in Europe, the two showed a median increase of 8 months in the overall survival of patients. So perhaps there is hope…
But if resminostat is only found to work in combination with sorafenib, what will that mean for the pricing of both drugs as an overall therapy?
In this scenario, it is also worth considering Transgene (France), which is currently in phase III trials for an oncolytic therapy also targeting HCC.
Meanwhile, 4SC is also doing other trials for different cancers (including NSCLC, Lymphoma and colorectal types) in Europe & Japan, where it has a deal with Yakult. Then it has a deal with Menarini for €95M for the commercialization of Resminostat in the Asia Pacific region, and secured €29M in funding in 2015.
4SC is betting big on worldwide approvals for their liver cancer therapy. Will future trials prove this epigenetic therapy as a new hope for patients?