Belgian TiGenix NV released the phase I results of their cardiac stem-cell therapy at the Congress of the European Society of Cardiology (ESC) in London earlier this month. In July, TiGenix acquired the smaller biotech CoreTherapix with the AlloCSC-01 pipeline. This stem-cell therapy is for regeneration of heart muscle following a heart attack, and is currently undergoing its phase II trial ‘CAREMI’.
 TiGenix NV, from Leuven (Belgium) recently bought out Madrid-based CoreTherapix, a former subsidiary of Spanish biotech Genetrix for a contractual total of €267M. This is a hefty sum for a small biotech only founded in 2008, for sure. However, it is the excitement surrounding Coretherapix’s cardiac stem cell therapy AlloCSC-01 which has sparked a euromillion rush.
TiGenix NV, from Leuven (Belgium) recently bought out Madrid-based CoreTherapix, a former subsidiary of Spanish biotech Genetrix for a contractual total of €267M. This is a hefty sum for a small biotech only founded in 2008, for sure. However, it is the excitement surrounding Coretherapix’s cardiac stem cell therapy AlloCSC-01 which has sparked a euromillion rush.
AlloCSC-01 is designed as a reparative tool to treat patients who have experienced an acute myocardial infarction (AMI) in order to reduce ischemic damage. Phase I results have been overwhelmingly positive, with AlloCSC-01 demonstrating a comprehensive safety profile (announced at the ESC). Since over 1.5 million AMIs occur annually across the US and EU, and the projected annual cost of heart failure in the US alone is set to rise to almost €63Bn, a repair treatment using stem-cells is of high interest in the biotech industry.
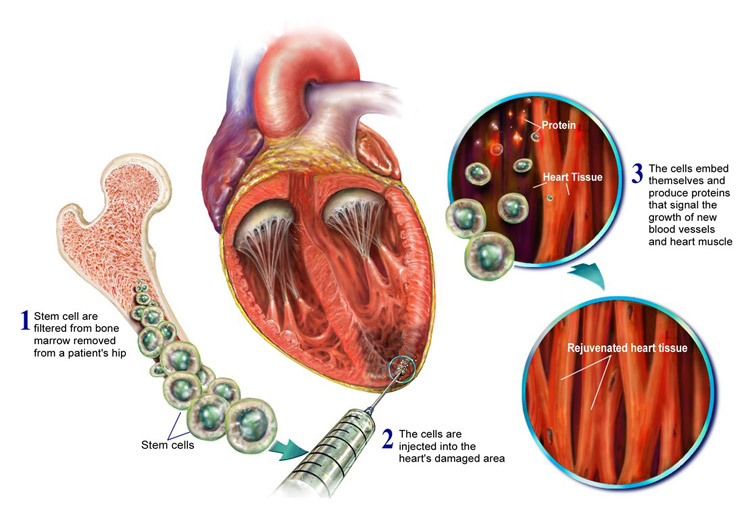
AlloCSC-01 is developed by harvesting of stem-cells from the patient’s heart and culturing them in vitro. Intracoronary injection of the stem-cells then activates endogenous regenerative pathways, reducing scarring and inflammation whilst promoting growth of new contractile myocytes.
The cardio repair European multidisciplinary initiative (CAREMI) project by CoreTherapix is now trialing AlloCSC-01 phase II in a total of 55 patients (49 new) across 8 locations in Spain and Belgium. CAREMI is expected to complete recruitment by the end of this quarter (since only 60% of the target number of patients are enrolled so far), with a 6-month interim efficacy analysis to be released in the second half of 2016.
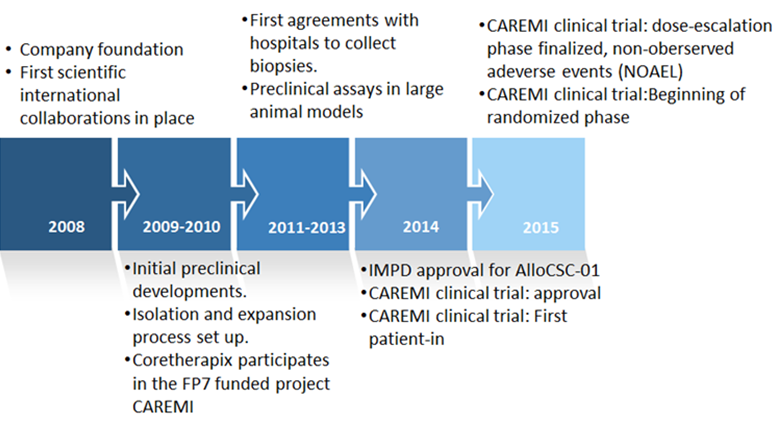
Since the only AMI treatments that exist at the moment are mostly palliative or to restore myocardial function (e.g. by angioplasty and insertion of a stent to support the vascular lumen), the focus of CAREMI trials is to use AlloCSC-01 to essentially reverse the damage altogether. It is therefore unsurprising that the project has received a total budget of €11.3M from the European Commission.
With the cardiovascular epidemic racking up billions in costs, this stem-cell therapy could therefore be a major breakthrough in the treatment market for myocardial infarction.