After the invasion of the London stock exchange by US-based companies PureTech (€147M raised) and Verseon (€92M raised) last June, it’s now the turn of two EU companies to go public in the British capital. Shield Therapeutics wants to grab a massive £110 million, while Faron Pharmaceuticals had not disclose its intention yet.
Faron, based in Turku (Finland), intends to fund its Phase III study of Traumakine, a drug in development for treatment of acute respiratory distress syndrome (“ARDS”). ARDS is a severe medical condition characterised by widespread capillary leakage and inflammation in the lungs, most often as a result of sepsis, pneumonia or significant trauma. With no current treatment, the disease is considered an orphan drug disease with a high, 30–45% mortality rate.
Traumakine -which development has been granted a €6 million financing from EU- has shown encouraging results in Phase I/II studies, with an associated 81% reduction in the odds of 28 day mortality rate in patients with ARDS.
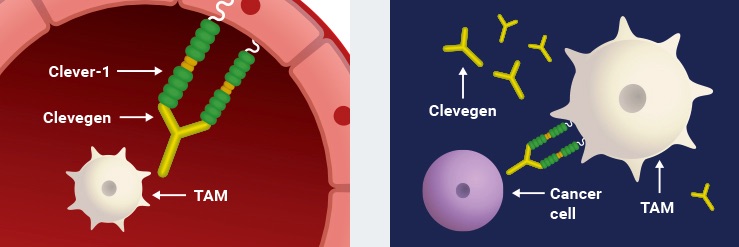
Faron’s pipeline also includes a novel pre-clinical cancer immunotherapy called Clevegen. The treatment consists of an anti-Clever-1 antibody, which converts the immune environment around a tumour from being immune suppressive to immune stimulating by reducing the number of tumour-associated macrophages.
On the other hand, Shield Therapeutics announces its intention to raise up to £110 million (€150M) in its homeland, the UK. The Gateshead-based company possesses two late-stage products in development: Feraccru, for the treatment of iron deficiency anaemia; and PT20, a novel phosphate binder that is being developed for the treatment of hyperphosphatemia related to chronic kidney disease .
Commenting on the announcement, Carl Sterritt, CEO of Shield Therapeutics, said: “We have a European application for marketing approval of Feraccru currently under review by the European Medicines Agency and have also completed a successful pivotal phase 2b study with PT20.”
Feraccru is expected to launch in Europe in 2016. With this drug on the market, Shield intends to generate enough revenue to sustain its own development.
In recent months, the London stock market has shown a growing appetite for Biotech companies. With this new bunch of companies on stage, the market will continue to heat up. Let’s hope this will not go to London Mayor’s head, who went crazy last June about floating a £10Bn biotech fund for London.