Humabs (Switzerland) has gotten some serious recognition for its antibody platform, as its candidates for Influenza have received FDA fast-track designation.
 A biologic therapy for Influenza A has been granted Fast Track Designation by the FDA, a ‘programme to accelerate the treatment commercialisation.
A biologic therapy for Influenza A has been granted Fast Track Designation by the FDA, a ‘programme to accelerate the treatment commercialisation.
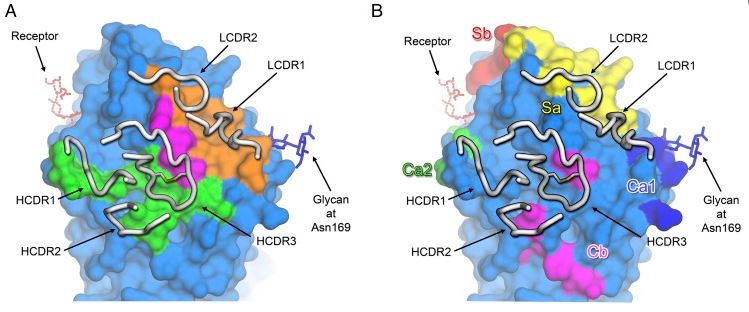
The therapy is based on an antibody (MEDI8852) that was initially developed by Humabs. This Biotech used human memory B-cells from patients that recovered from influenza to isolate an antibody with broad influenza-neutralising properties.
This idea of studying the immune system of people who overcame the targeted disease is the basis for their drug discovery platform, Cellclone, which allows Humabs to discover antibodies quickly. These antibodies sometimes target previously unknown or multiple mechanisms in infectious diseases.
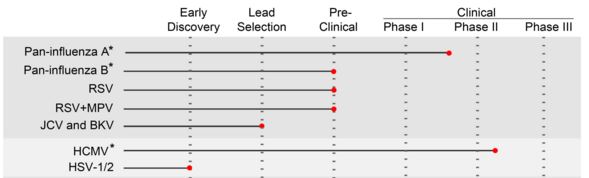
Besides Influenza, Cellclone was also used to develop antibody therapies for the infamous Ebola and Middle East respiratory syndrome (more commonly known in the news as ‘MERS‘).
MEDI8852 is being developed by MedImmune, the biologics development arm of AstraZeneca. It is currently in phase Ib/IIa trials, after showing acceptable safety levels in a phase I trial
This antibody binds to a highly conserved region of influenza A strain sequences, meaning that it could still be effective with different types of virus (as the virus mutates with time). Therapies becoming ‘outdated’ by quick mutation rates is one of the challenges in developing treatments which tackle influenza.
The new Fast Track designation should help MedImmune with the future development of this particular drug.
Additionally, it also lends additional credibility to Humab’s platform, which is being used to develop therapies for other infectious diseases, such as pneumonia, hepatitis B and rabies. There are also potential applications in inflammatory diseases and cancers (through immuno-oncological approaches).
FDA’s Fast Track is still not that common for Infectious Disease, so this milestone is great news for both MedImmune and Humabs.