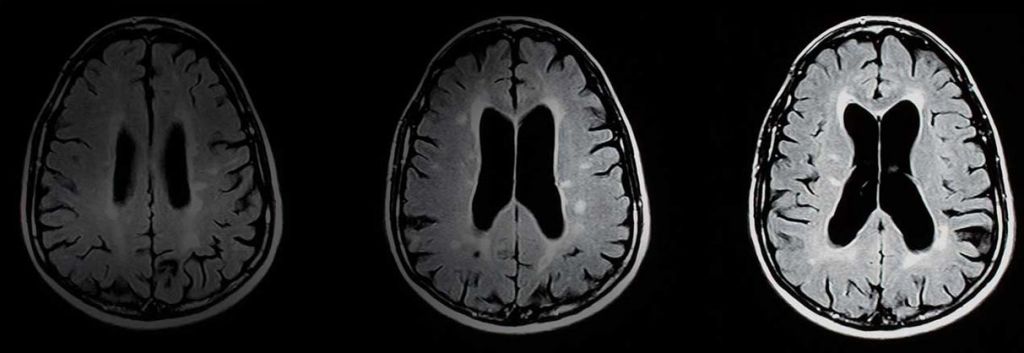
The progressive aging of the population in developed countries is turning Alzheimer into one of the biggest medical challenges of our times. And it looks like it is going to remain an unresolved problem for the moment, as Roche’s candidate failed its last clinical trial. The drug, developed in collaboration with German Evotec, didn’t meet the primary endpoint of its phase IIb.
The candidate, Sembragiline, belongs to a family of inhibitors of monoamine oxidase type B (MAO-B), which are typically used to treat Parkinson’s disease. The enzyme breaks down the chemical messenger dopamine in the brain and contributes to the production of free radicals. These free radicals cause oxidative stress and, subsequently, contribute to the pathogenesis of the Alzheimer disease. For these reasons, the selective MAO-B inhibitor is targeted to treat Alzheimer’ symptoms and potentially slow the disease’s progression.
However, and despite the preliminary analysis that showed the safety of the drug, Sembragiline failed to demonstrate any benefit on the primary endpoint after 52 weeks of treatment. Roche has initiated a process to evaluate all secondary endpoint read-outs on the candidate and to consider all further development options.
Roche is joining the list of companies that tried and failed to develop the long-awaited treatment to Alzheimer’s. Both solanezumab and bapineuzumab, developed respectively by Eli Lilly and the partnership J&J-Pfizer, failed in pioneering phases III. The unpromising results, however, didn’t discourage the big pharmas, that attributed the data to the advanced state of the patients that were tested in the trials. With so many families suffering from the devastating symptoms of Alzheimer, the patients keep clutching at straws.
Same goes to investors, according to the stock market movements. The most notorious story is, without any doubt, Axovant’s impressive IPO. The company raised €284M just after acquiring GSK’ scrapped project on Alzheimer for a few millions (€4.5M).
Roche and Evotec’s results are probably pretty bad if the companies aren’t releasing any data at all from the clinical trial. The Swiss giant is considering all its options, probably looking for another target population. But with these discouraging outcomes, they better save their money for something more promising.