Sanofi Genzyme (Boston and France) have obtained good phase I/II results with a candidate for the rare Pompe Disease and expects to move to phase III in the second quarter of 2016.
 Sanofi Genzyme continues to bet on rare diseases and neurodegenerative disorders, now with a therapy for late-onset Pompe Disease.
Sanofi Genzyme continues to bet on rare diseases and neurodegenerative disorders, now with a therapy for late-onset Pompe Disease.
Pompe Disease is a neuromuscular condition caused by a dysfunction in acid alpha-glucosidase (GAA), a lysosomal enzyme that is key to break down glycogen (a common sugar ‘storage’ molecule in muscles). Without the enzyme, glycogen accumulates and damages the muscles.
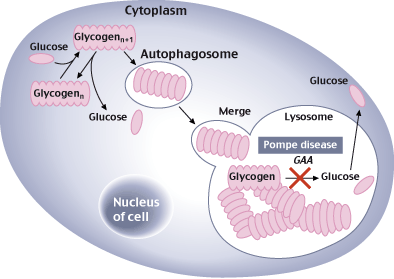
This condition can affect babies (early onset) or develop later in life (late onset). Though the prognosis improves with later onset, Pompe is a debilitating disease and patients experience difficulties with walking and breathing.
Treatment involves enzyme replacement therapy (ERT), and Sanofi Genzyme is developing a second-generation therapy (neoGAA) based on new engineered enzymes.
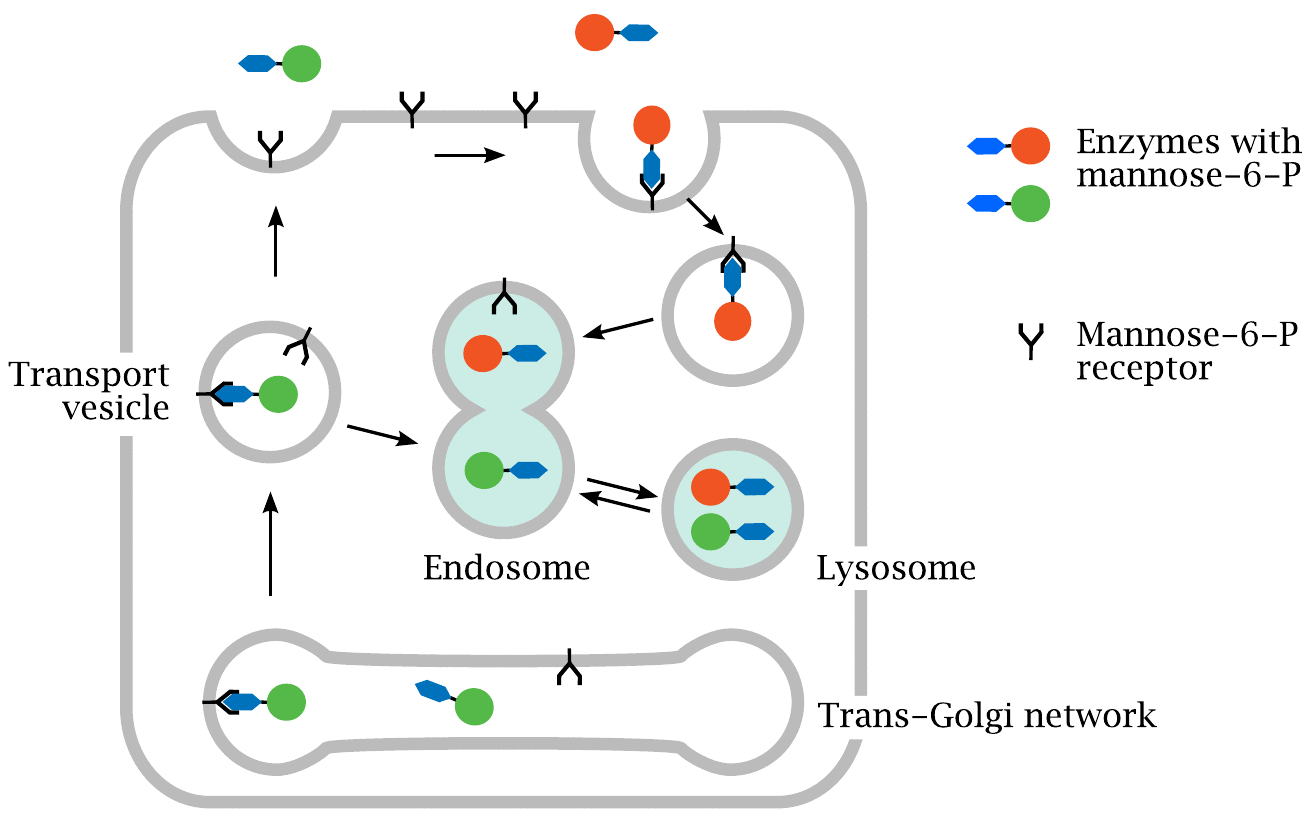
These enzymes have a greater affinity for M6P receptors (a type of cellular ‘gate’ for lysosomal proteins) found on muscle cells. This is expected to improve the clearance of glycogen in muscle cells.
Sanofi Genzyme is now presenting the data from the phase I/II trial (NEO1), which enrolled 24 patients and has shown good safety and tolerability results.
The Biotech finds these results a positive proof of concept for neoGAA, and certainly encouraging enough to already plan a phase III trial for the second quarter of 2016.
Genzyme is a specialist of enzyme replacement treatments. One of its first drug was Crrezyme, also an enzyme replacement, which was for long the most expensive drug worldwide (€200,000/year). This drug was prescribed for a life time and was generating massive return on investment for Genzyme.
This new enzyme replacement therapy for Pompe’s disease could have the same outcome, if the Phase III is successful.





