CAR-T is the way forward in Cancer therapeutics, which is one of the leading causes of Mortality in the Western world. Now this European consortium has amassed €6M for a more unified approach to CAR-T under a European Union Commission program.
 In response to this major health issue Miltenyi Biotec (Germany) coordinates the new EU-funded project CARAT taking on the mission to deliver new methods to enable the wide use of novel personalised cancer treatment.
In response to this major health issue Miltenyi Biotec (Germany) coordinates the new EU-funded project CARAT taking on the mission to deliver new methods to enable the wide use of novel personalised cancer treatment.
The project consortium comprises a multi-national team of leading experts from eight European partnering institutions, which include biotech and research centres in Germany, Italy, UK (and Wales) and France.

Equipped with a total budget of €6M, CARAT aims to integrate innovative cell manufacturing tools and enabling technologies into a new, comprehensive platform that will facilitate the safe, automated, and cost-efficient manufacture of highly effective so-called CAR T-cells.
Andrew Kaiser, scientific coordinator of the CARAT consortium at Miltenyi Biotec, explained that the goal of the program is to get CAR-T manufacturing process and research tools working together in order to overcome barriers to development through collaboration.
Our vision is to elevate cellular therapies to the next level in a cost-effective and easy-to-handle process that can be readily upscaled and disseminated worldwide“
Recent success stories of cancer therapies based on these so-called CAR T-Cells have raised enormous scientific and public expectations to cure severely ill patients. In latest studies, including those by Cellectis (France), such T-cell treatments have proven to be very successful – with up to 93% response!
Another example is a clinical trial of a highly aggressive form of acute lymphoblastic leukaemia (ALL) at the University of Pennsylvania in collaboration with Novartis (Switzerland), where 89% of children and adults showed no evidence of cancer after receiving a CAR T-cell therapy.
Consequently, the FDA designated T-cell treatment, CTL019, as a “breakthrough therapy” in 2014 for relapsed and treatment-resistant acute lymphoblastic leukaemia in adults and children. Read our more extensive review on CAR-T and how it works for a better understanding.
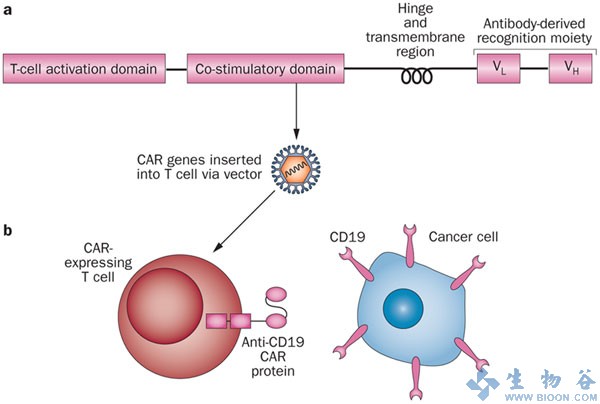
However, translation into clinics and broad application of such advanced personalised cell therapy is currently hampered by the technologically complex, costly and error-prone procedure of manufacturing gene-modified T-Cells for each patient individually.
And when we interviewed the CEO of Cellectis, Andre Choulika, he surprisingly said that CAR-T was not the miracle cure for cancer, despite this billion Euro Biotech recently making a breakthrough in research surrounding CAR-T control (amongst other milestones).
So what will be the next step? And will other European CAR-T specialists join the CARAT team?





