How can you build the best case for a new drug application to the regulatory agencies? Preclinical studies are a crucial part in determining the pharmacokinetics of a drug – and more specifically how it is going to interact with drug transporters. So reaching out to the leading provider and consultant in this area (with strong academic roots) is the logical step at this stage of development.
Measurement of the potential of new drugs to be substrates or inhibitors of drug transporters is important in evaluating the safety of a drug in the preclinical development process, as well as to predict potential pharmacokinetic based interactions with other medicines.
Transporters have become increasingly important in drug development due to the major role they play in absorption, distribution and excretion of endogenous and exogenous compounds. The FDA & EMA emphasise the importance of evaluating new drug candidates for transporter-mediated drug-drug interactions (DDI) with a determination of substrate and inhibition potential.
Experienced Contract Research Organizations (CRO) are positioned to help ease the process for developers, in order to help get their drug to an FDA standard.
Sekisui XenoTech is one such contract research company – positioned perfectly with core focus around in vitro preclinical definitive models (GLP / non-GLP). Specifically, they are known as a leader in the field of Drug Transport, Drug Metabolism and DDI.
Sekisui XenoTech performs in vitro transporter studies to determine if compounds are substrates or inhibitors of clinically-relevant transporters using validated and industry accepted test systems.
Access to the most extensive library of transporters & assays in the world makes them an easy choice for biotechnology and pharmaceutical companies worldwide.
The team of professionals and researchers at Sekisui XenoTech are also publishing novel information on drug transporters.
For example, they recently published a paper on the ability of the anti-fungal ketoconazole and its alternative clinical CYP3A4/5 inhibitors to inhibit key drug transporters. Ketoconazole has long been used as a clinical CYP3A4/5 inhibitor, however, it is known to result in adverse effects which include liver or adrenal insufficiency.
Because of this, the EMA & FDA recommended against the use of ketoconazole as a clinical CYP3A4/5 inhibitor in 2013, and specifically recommended the use of other compounds with less severe side effects – such as itraconazole, clarithromycin and ritonavir.
Although the pharmacokinetic profile for the interaction of these compounds with Cytochrome P450 is well documented, there was little information about their potential to inhibit drug transporters.
Generally, the interactions between drugs and transporters are not well known. So testing the potential for drugs to be substrates or inhibitors of drug transporters can help predict drug-drug interactions (e.g. using ketoconazole as a model example), and can therefore help drug developers make an informed decision.
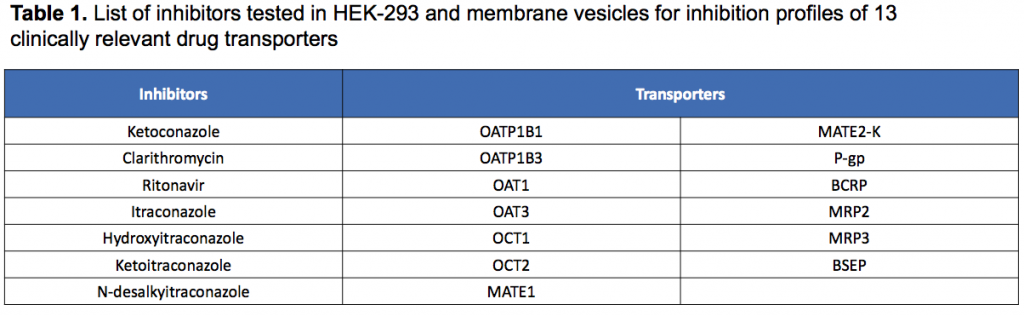
In the paper (which is freely available), Sekisui XenoTech have established the inhibition profile for ketoconazole, ritonavir, clarithromycin, itraconazole (and others) against 13 clinically relevant drug transporters:
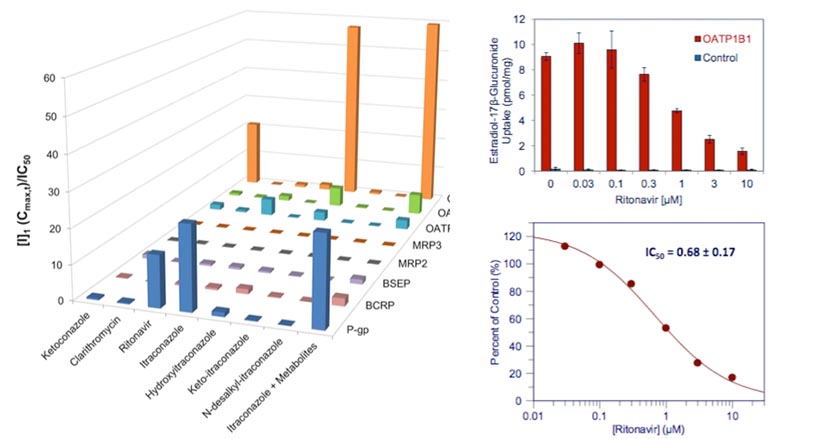
It was found that none of the alternatives to ketoconazole provided a clean inhibition profile towards all 13 drug transporters (each compound has a unique drug transport inhibition profile). Therefore, the best choice for a strong clinical CYP3A4/5 inhibitor depends on the unique transporter substrate profile of the drug candidate.
To learn more about their latest paper, Sekisui XenoTech also offers a complimentary webinar, poster and abstract – all of which you can find on their website.
Sekisui XenoTech has over 22 years of ADMET, PK and DDI experience, with a proven track record of helping biotechnology and pharmaceutical companies globally work through the preclinical drug development stage to FDA approval through study design, conduct and interpretation.

To put this into context, 36 of the Top 40 Pharmas in the world have turned to Sekisui XenoTech for their services. In addition, they even assure a free-of-charge repetition of services should it be needed.
Find out more about XenoTech’s Drug Transporters
Feature Image Credit: © Dolgachov (BigStock ID91831535)