By Michael Arciero, vice president of intellectual property and corporate development, ERS Genomics

CRISPR/Cas9 gene editing technology is a patented invention, so any commercial use requires a license. Despite the confusion caused by several high-profile patent interference cases, the patent landscape for CRISPR/Cas9 is clearer than you may think.
Here are the 5 key facts you need to know, so you can secure the licenses that will protect your product and your company.
1: If you’re using CRISPR/Cas9 in the U.S., a Broad license may not be enough
There has been some understandable confusion regarding CRISPR/Cas9 patents in the U.S. following the high-profile patent interference situation between the Broad Institute and the University of California, the University of Vienna, and Emmanuelle Charpentier (collectively known as CVC).
Although some patent disputes are still ongoing between these two organizations, the overall take-home message is not as complicated as it may seem. The US Patent Trial and Appeal Board (PTAB)-initiated interference between CVC and the Broad – which is currently under appeal – only applies to certain CVC applications with claims limited to use of CRISPR/Cas9 in eukaryotic cells.
The CVC portfolio has more than 55 patents still upheld in the U.S., covering the use of CRISPR/Cas9 gene editing in all cells (including eukaryotic cells).
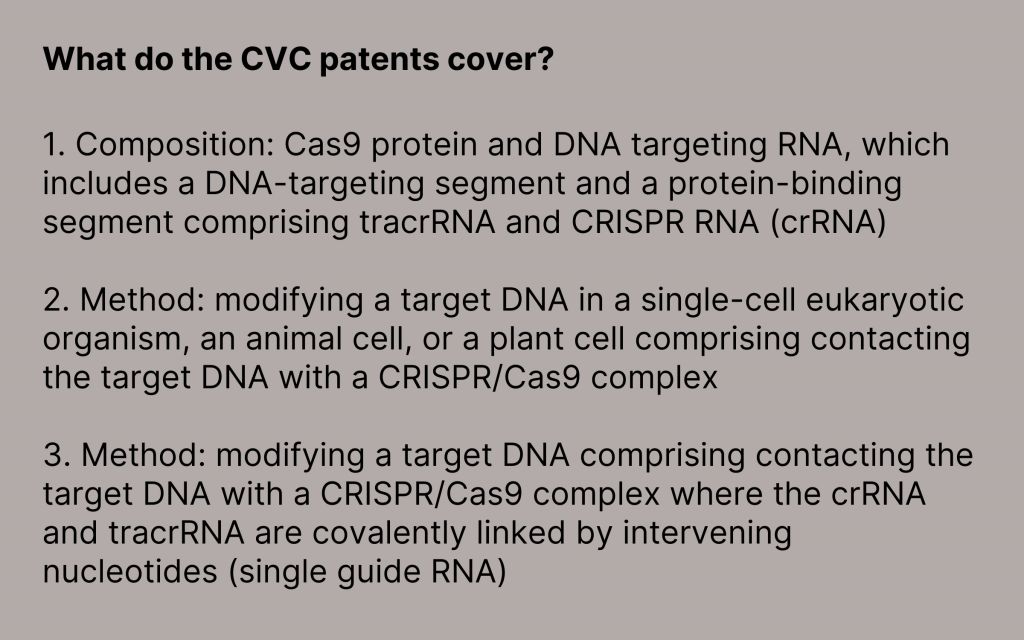
These foundational patents cover the very basis of CRISPR/Cas9 technology, including both methods (the technical steps required to perform gene editing) and composition (the necessary reagents), and are not at issue in the interference actions.
This means that if you are based in the U.S. and using CRISPR/Cas9, you need a CVC license. You may also need a license from the Broad institute if you work specifically in eukaryotic cells, but you’ll definitely need one from CVC to start with.
2: The CRISPR/Cas9 patent situation is clearer outside the U.S.
In Europe, CVC holds the broadest foundational patents on the use of CRISPR/Cas9 gene editing technology. The Broad Institute’s initial patents in Europe have been revoked or substantially narrowed, with no opportunity to overturn the decision.
CVC holds patents in Europe covering uses of CRISPR/Cas9 in both cellular and non-cellular settings and remains the only CRISPR/Cas9 patent estate to survive opposition in the region.
CVC also holds patents in other key markets worldwide, including Canada, China, Brazil, and Australia, and this is continually expanding. In 2021, the Japanese patent office rejected opposition to a CVC patent, reaffirming the patentability of the technology. More recently, CVC has been granted a patent in India, covering methods and compositions for use of CRISPR/Cas9 gene editing in all cell types.
Over 100 patents are currently held by CVC globally. Companies planning to sell products or services involving CRISPR/Cas9 gene editing technology are highly likely to need a license from CVC in most parts of the world, making ERS Genomics the go-to global licensing office for CRISPR/Cas9.
3: One CRISPR/Cas9 license is unlikely to be enough
There are many different components to CRISPR/Cas9 gene editing technology, from the underlying reagents (composition) to the way the technology is used in the lab (methods).
This means that one license is probably not enough.
It may sound complicated, but this situation is commonplace in the modern world. For example, devices like your phone or your laptop contain many different patented components, technologies and processes, often licensed from different providers.
CVC has over 100 different patents covering these different aspects of the technology, from the single guide (sg) RNA to modifications in trans-activating crispr RNA (tracrRNA). While ERS Genomics makes the full suite of CVC patents available in one package, additional licenses may be required for the use of CRISPR/Cas9, particularly for use in eukaryotic cells or for certain niche applications.
IP lawyers are used to dealing with these situations and can tell you exactly what you need to safely use CRISPR/Cas9 for your purposes.
Ultimately, the foundational patents held by CVC cover the fundamentals of the technology, including the use of sgRNA as well as dual guide RNA.
4: Whatever happens with the patent interference case in the U.S., you still need a CVC License
With appeals still working their way through the U.S. legal system, you might be tempted to bury your head in the sand until it’s all over. But this would be ill-advised.
CVC has more than 55 issued patents that are not subject to ongoing interference, including foundational patents covering the use of sgRNA, modifications to tracrRNA, and the use of CRISPR/Cas9 in all cell types.
The interference has no bearing on these foundational patents, so waiting for it to resolve is not a valid strategy. Whatever the outcome of the U.S. legal battle, you will still need a CVC license to use CRISPR/Cas9 in your commercial applications.
5: The risks aren’t worth it
Given the high media profile of these legal disputes, claiming ignorance if you don’t have a license for using CRISPR/Cas9 is going to be difficult. And with willful patent infringement warranting treble damages in the U.S., it just isn’t worth the risk.
If you’re a startup or fledgling company, or an investor with funds in biotech startups, getting a license in place from the outset shows you’re serious about taking your product to market and plan to succeed.
Larger pharmaceutical and biotech companies may be more likely to have a dedicated legal team to focus on the licensing issue, but don’t assume it has been dealt with. These organizations are also likely to be gambling with bigger investments of time, money, and labor, so it’s a false economy to skimp when it comes to licensing the technology.
The bottom line is that using CRISPR/Cas9 without a license calls the legitimacy of your research, and your product, into question. Getting the right licenses in place from the start can protect you and your company from costly litigation and damaging publicity further down the road.
CRISPR with confidence
Whatever your application, if you are using CRISPR/Cas9 gene editing technology you most likely need to license a CVC patent to do so. The 100-plus global CVC patents cover the very foundation of this game-changing technology, as well as more recent innovations, and is the first step to using CRISPR/Cas9 with confidence.
Legal disclaimer: This article covers the use of CRISPR/Cas9 gene editing technology for all cases except use as a direct human therapeutic. It does not constitute legal advice and we advise speaking to an IP expert regarding your specific application.