The rapid spread of Covid-19 around the world has left drug developers with limited time to identify drug candidates to fight the virus. Amid this crisis, artificial intelligence has proven its value as a tool to make drug discovery faster and cost-effective.
Drug discovery is an expensive, long-winded process. Taking a drug from discovery to market can require 10 to 12 years and cost over €2B. Still, less than 12% of clinical-stage candidates gain approval. Additionally, patents on therapeutic compounds are limited to 20 years from the date of filing, meaning companies have at best 10 years of exclusivity on their product before generic versions can be produced by competitors.
Despite showing signs of stagnation until fairly recently, it now appears drug discovery has gained a new superpower. It hasn’t been bitten by a radioactive spider, doused in cosmic rays or struck by lightning, but it has gained a nifty new upgrade to its super suite of tools: artificial intelligence. Using techniques such as machine learning or deep learning, artificial intelligence can help speed up the drug development process by identifying therapeutic candidates, either from existing drugs or brand new compounds.
While a number of companies have been developing AI tools for drug discovery for several years, the field is still rather young. The first European drug candidate identified using AI only entered clinical trials earlier this year. However, as the largest global pandemic since the Spanish flu sweeps the world, AI has seen its moment to shine. Several players in Europe have used their AI tools to identify potential treatments for Covid-19 faster than ever before.
Shaving years off the route to market
We have learned faster than ever before about the SARS-CoV-2 virus upon its outbreak than about any other before it. This is in part due to advancements in rapid genome sequencing technologies. Artificial intelligence can then use this information to predict which compounds are most likely to be effective against a specific target. But for this to work, the quality of the data is essential.
Exscientia, based in Oxford, has a two-pronged approach to drug discovery: an in-house lab for data generation and experimentation, and a machine learning platform for designing experiments.
“It’s driven by data,” explained Andrew Hopkins, CEO of Exscientia. “As a company, we focus on generating high-quality data to seed our algorithms and that’s an important aspect of what we do as well. We are building machine learning models, which are then used for dry design.”
“One of the key features of our approach is that we’ve been able to demonstrate we can discover and optimize molecules to drug candidates far more quickly than other people have been able to do, on the order of 5 to 10 orders of magnitude improvements.”
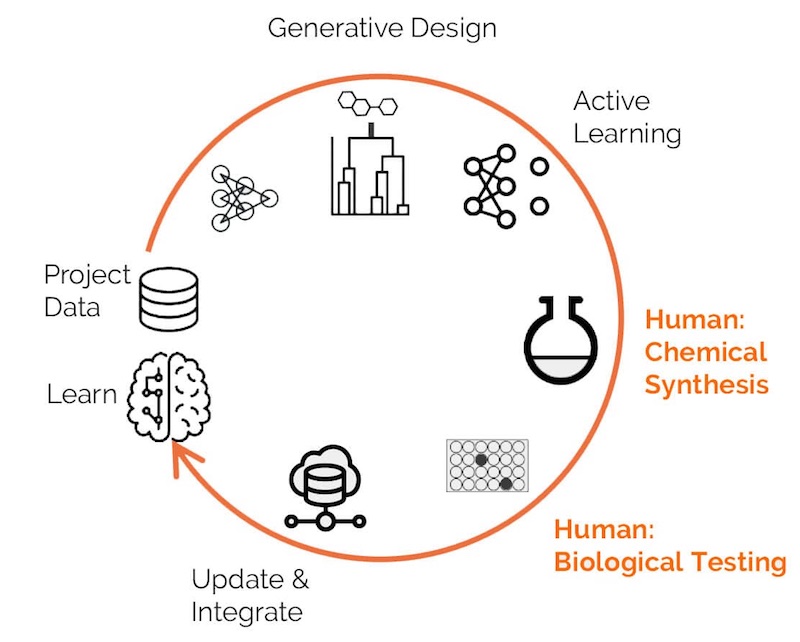
Exscientia is collaborating with Diamond Light Source and the Scripps Institute to identify potential Covid-19 therapeutics from a massive databank of 15,000 potential candidates, including approved as well as investigational drugs. All these drug candidates had previously passed the human safety testing stage. The team can rapidly identify treatment candidates, which can then be fast-tracked to be tested directly in Covid-19 patients.
Earlier this year, Exscientia’s drug candidate for obsessive-compulsive disorder was the first AI-designed drug to enter clinical trials, doing so within just 12 months of identification. Typically, going from identification to the clinic is a five-year process.
Another player shaving years from the journey to the clinic is French company Iktos. Its deep learning technology created computer models that can learn new tasks, such as the design of new molecules, by accessing public databases. The company has partnerships with Merck KGaA and Servier that validate the potential of this technology to increase productivity in the drug discovery space through predictive modeling and design. In March, Iktos partnered with SRI International to discover new compounds against multiple viruses, including SARS-CoV-2.
Identifying unusual drug candidates
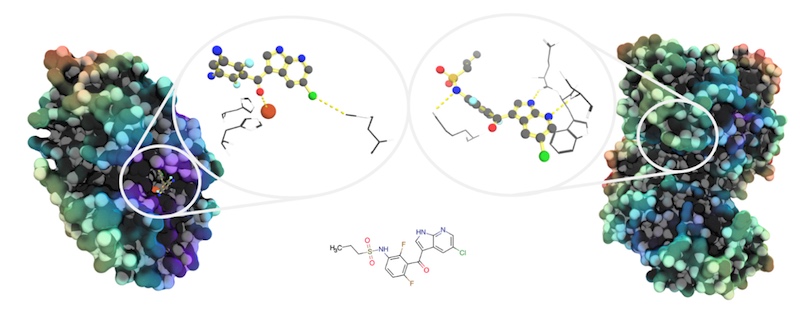
Artificial intelligence methods have already yielded results for two companies. British drug discovery company BenevolentAI and Germany-based Innoplexus identified potential therapeutic options for Covid-19 patients earlier this year.
BenevolentAI’s approach integrates many types of data to generate predictive models for a wide range of diseases, which are in turn improved with feedback from experimental data.
“Recreating representations of disease-relevant mechanisms has required the integration of protein network biology, disease, biological processes, tissue, cell line, pharmacology, multi-omics and clinical data from public and commercial resources,” explained Mark Davies, Senior Vice President of Biomedical Informatics at BenevolentAI.
“We are also very aware of the gaps in public datasets and ontologies, and have adapted our workflows to allow for the introduction of proprietary definitions of important biomedical entities to ensure we have the most accurate representation of the biological systems.”
While the company focuses on neurology, immunology, oncology, and inflammation, early into the pandemic the team wondered if its technology could be applied to find treatments for this novel disease.
“The short answer was yes. A small team working over a timeframe of 48 hours identified baracitinib as a potential treatment for Covid-19 patients. Based on this work, baracitinib is currently being assessed in more than 12 clinical trials worldwide, including large global trials by the NIAID and Eli Lilly,” said Davies.
Innoplexus uses a similar approach but with a broader data input consisting of published papers, online data from presentations, symposiums and conferences, clinical trial data, and publicly available hospital data as well as unpublished datasets. In March, the company released an analysis of potential Covid-19 therapeutics as well as drug combinations.
“It covers the real-world evidence of the patient-level data from hospitals, from different data-generating companies, and from biobanks. It is all the human data available in the known universe,” explained Gunjan Bhardwaj, CEO and founder of Innoplexus.
“A lot of our predictions were not from the viral treatment domain. There were completely counterintuitive combination options that we have suggested, which you can never identify using only the Covid-19 data,” he added.
Hydroxychloroquine, the anti-malarial drug touted by US President Donald Trump and Brazilian President Jair Bolsonaro as a miracle cure, was identified by the Innoplexus platform but later shown by several clinical trials to have no effect. The company also noted chloroquine, another anti-malarial, may work in combination with Pegasys (hepatitis C), tocilizumab (rheumatoid arthritis), or remdesivir (Ebola). The latter has since been approved for treating Covid-19 patients.
For its part, Cambridge-based AI VIVO predicted that the commonly used drug dexamethasone could help Covid-19 patients weeks before clinical trial results confirmed it.
Data curation and the future of AI in drug discovery
Many of the artificial intelligence platforms for drug discovery can be applied to almost any indication. One of the biggest limiting factors however is the quality of the data, including meta-data. That is, the information about how, where, and when data was obtained.
One company looking at cultivating better data collection in the field is Synthace, a British company creating computer-aided biology software. The use of this kind of technology has been boosted during the pandemic, as labs around the world increasingly needed to allow remote work.
“Whilst there has been a lot of discussions around the applications of these techniques, to date there’s been little discussion around how that data can be generated in an intelligent way — such that we can really see a data flywheel for wet-lab biology rather than the current hamster wheel powered by human data wranglers,” said Peter Crane, Corporate Strategy Manager at Synthace.
“Determining what data and meta-data to capture, and in what level of abstraction, is essential in this regard. We believe experimental tools that holistically combine intelligent experimental design and automation, as well as meta-data capture and association will be important in bringing the power of these techniques into the laboratory environment,” he added.
Adopting automation helps improve data quality, but this adds another barrier to accepting technology that some researchers are not yet ready for. Software solutions and the AI approach are already difficult to stomach for some.
“Whether it is with scientists and researchers directly using the systems or with senior leadership of companies who are considering investing in AI approaches, we need to ensure they have the necessary information and justification to embrace the change,” said Davies.
“Having more examples of AI approaches demonstrating a positive impact within the drug discovery process will only help to improve adoption and reduce the acceptance barrier.”
With success stories from the Covid-19 pandemic, the future seems promising for artificial intelligence in drug discovery, but this superpower could have its kryptonite too. One issue revolves around data security and patient privacy, currently hot topics of discussion within the field. Another is that AI requires access to robust, high-quality data. This means that, in the short term, only fields with a high volume of good quality data will benefit from AI-enabled platforms.
Cover illustration by Elena Resko. Images from Exscientia and BenevolentAI.





