Newsletter Signup - Under Article / In Page
"*" indicates required fields
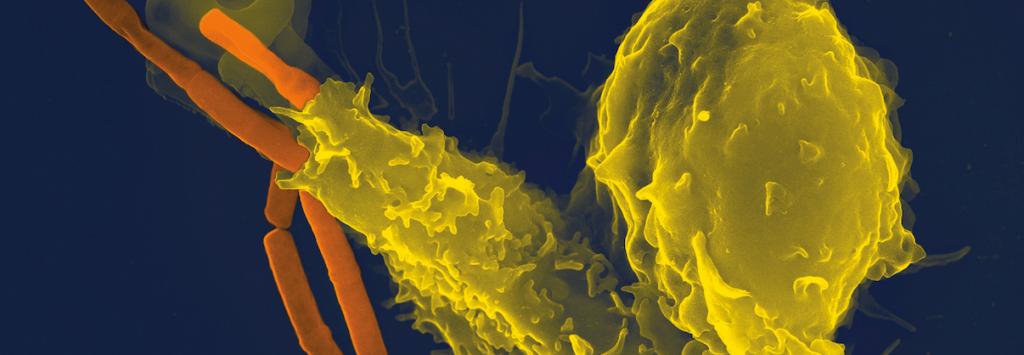
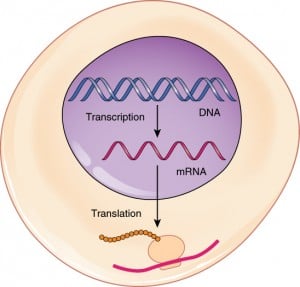
So for those who don’t know what a mRNA is, here are some basics. This diagram below shows the transcription/translation mechanism in protein synthesis that occurs in Eukaryote cells, where mRNA plays a crucial role. There’s a more detailed artist’s impression on this process by the Wellcome Trust here.
The first therapeutical description was back in 1992, which considering how major mRNA is to understanding Cellular biology today, is surprisingly recent. So this is a very fresh sector which has been exceptional in its development, and has therefore generated a lot of excitement recently.
Targeting single molecules of mRNA before it produces proteins responsible for certain diseases is a strategy considered less dangerous than editing at the gene level (e.g. insertional mutagenesis). It is also thought this process could be better tolerated by the human body than using recombinant protein manufactured by bacteria.
That’s two reasons why so many Biotech start-ups are therefore betting on this technology. But there is still much more to this argument which we can discuss….
So how does one create therapeutic mRNA? And then what can we do with it?
Basically every mRNA project starts with a DNA sequence that is coding for a deficient protein, or an antigen produced by an infectious agent (bacteria, viruses etc.) or a tumor in the case of cancer.
Then the complementary mRNA (a copy of the strand) is produced within the cell too. This is the stage when industry competitors develop a proprietary version for therapeutic research and development, each with its unique advantages and limits.
Application of this technology can be segmented in three majors:
- Infectious diseases vaccination, mRNA provides better immune response than the one achieved with recombinant protein coupled with adjuvants,
- Cancer vaccination, possibility via Immuno-oncology and personalized treatments,
- Therapeutic use: Thanks to the mRNA, the cell will be able to express a protein that was deficient (e.g. functional CFTR channel in Cystic Fibrosis).
Using mRNA to get to the route of the problem before a protein is translated is a more cost-effective strategy compared to Cell therapies like CAR-T. But in comparison to CAR-T, mRNA Immuno-oncology clinical trials behave toward good results rather than accurate data, but hey, it’s still early days in this clinical field!
Back to business: How is such a small molecule delivered to a patient?
The administration of the mRNA uses several techniques including injection with vectors, nebulisation in lungs or electroporation under the skin. All depends on which organ you are targeting. For example:
- To produce an antigen for an immune response (e.g. in Immuno-oncology), it seems logical that immune cells are targeted.
- To produce a functional protein it is better to only target the specific organ affected (e.g. muscle in for a muscular dystrophy).
Type of vector used:
As always in Gene therapy, it can be viral (such as an Alpha virus) or non-viral (Liposome or cationic polymer). For mRNA, non viral vector is the big trend. There is also research into new Nanomedicine alternatives to liposome vectors, such as ‘cubosomes’, or using the silica shells of oceanic algae.
Who Moved First to Profit from this Trend’s Potential?
This is a snapshot of the three main players in the World (two German companies and Moderna from Boston in the US):
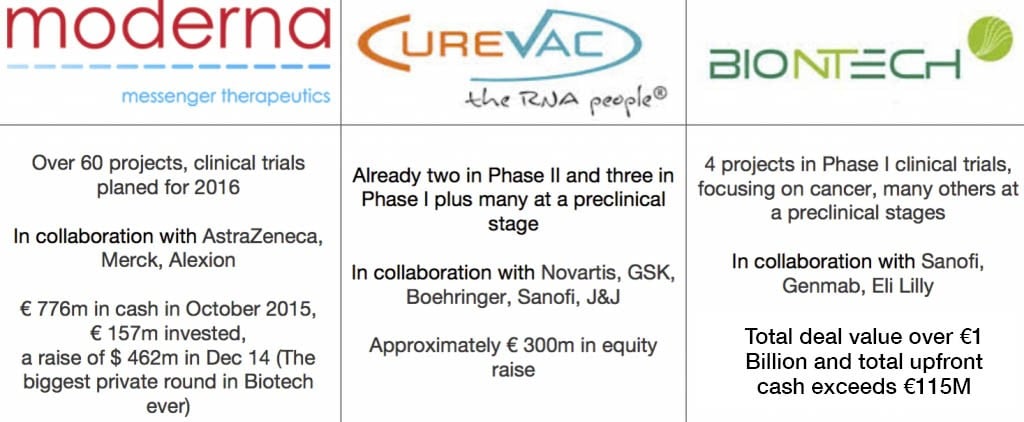
Companies such as these seem more advanced than others as they have platforms of development AND in house GMP manufacturing facilities. This naturally is attractive for potential collaborators and customers.
Asides these major players though, the rest of the field (perhaps a dozen other Biotechs) appears still restricted to the earliest stage of development. Some just focus on delivery, which is probably the biggest challenge. But of course, there is also a lot of reward and competition over mRNA research in Academia too.
So back to our Original Question: How could mRNA Overtake Biologicals?
There are many reasons which push this technology above other biological products in its three domains, for example:
- Compared to a complex protein or (even more challenging) – a genetically modified cell, a “simple” RNA strand seems easier to produce and characterize
- There is a better immune response as antigens are produced in the host cell: Specifically, mRNA is presented through the MHC I and II, which therefore induce a CD4+ (humoral) and CD8+ (cellular) immune response. Recombinant vaccines lead mostly to a CD4+ response…
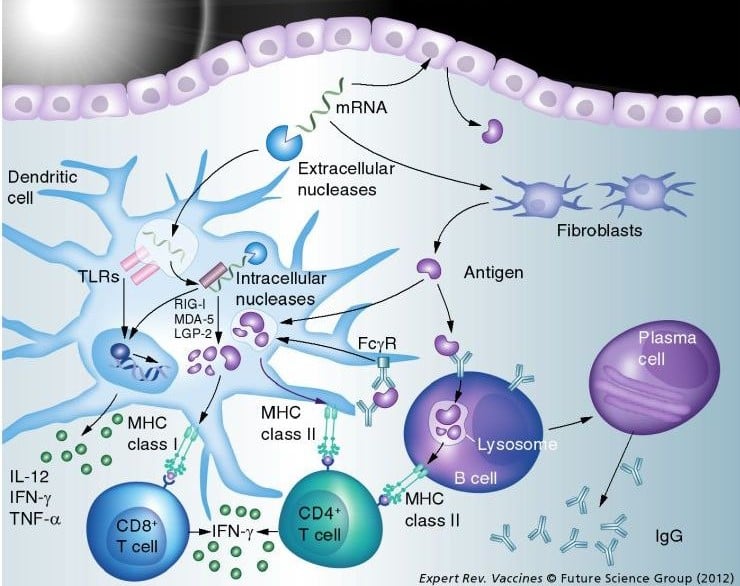
- With such technology, once delivery systems will be mastered, only targeted cells will express the protein, which seems better than injecting a recombinant protein into the blood
- Treatment with mRNA can be personalized but compared to Cell therapy, the time and cost of production is far lower… (a problem faced by many Biotechs – such as Cellectis and Israeli Pluristem – working on such challenges).
- Quick response for infectious diseases: Once a relevant DNA sequence is characterized, a vaccine can be produce within 10 days! For influenza, the production of vaccine with eggs take over 6 months…
So this technology seems to have great potential, despite the relative low maturity of the technology (no products available so far). So what are the current limits?
Here are major challenges to be overcome to rise this technology above others…
- Toxicity:
- mRNA can be immunogenic, which is great for vaccines. Regarding therapeutic mRNA, this sort of irritation has to be avoided. Integration of nucleoside-modified uridines helps to decrease immune reaction.
- Most part of vectors are recognized as foreign particles and an immunogenicity can be quick developed. Electroporation is one solution to this, as the mRNA is injected without an irritating vector.
- Because mRNA generally doesn’t replicate in the target cells (depending on vectors), thus its expression is limited in time.
- As it is immunogenic, multi-dose therapies are hard to perform. Moderna claims great improvement in this sector though…
- Stability of the mRNA: If today we can analyze DNA from Egyptian mummies, RNA is much more sensitive to heat but also to Ribonuclease, which is pretty much present everywhere.
- The delivery and targeting: That’s the problem folks! So far, targeting organs is possible through direct injection (e.g., intra-cardiac), otherwise vectors mostly end up in the liver. This step is crucial. As a matter of proof, around 25% of conferences we attended on mRNA were discussing delivery…
Also an improvement that can be highlighted with this technology is the regulation of mRNA expression: While developing system close to the TET-ON system (Display in the video above), MIT has successfully controlled the expression into a cell by simply adding a small, FDA-approved, chemical molecule.