CAR-T cell therapy has been able to effectively treat cancer in some patients for whom all other treatment options were exhausted. However, the technology still has a long road ahead before becoming a widespread treatment option. Philip spoke to Christian Homsy, CEO of Celyad, at our Refresh Meetup in Brussels about the challenges CAR-T cell therapies face.
What if we could recruit our own immune system to fight cancer? Well, CAR-T cell therapy does just that by genetically engineering T cells in order to boost the body’s immune response and destroy the tumor. This approach has great potential in treating cancer; Kymriah, the first approved CAR-T cell therapy, has shown cancer remission in 81% of patients for which other treatments had not worked.
However, the treatment comes with severe side effects that have caused patient deaths in clinical trials. It is also very expensive, with prices for CAR-T treatments soaring up to $475,000 (€400,000) per patient in acute lymphoblastic leukemia (ALL). In addition, the range of cancers the therapy can target is currently very limited.
The biotech industry is already working on circumventing these problems and making CAR-T cells more accessible for a wide range of cancer patients. Let’s have a look at how.
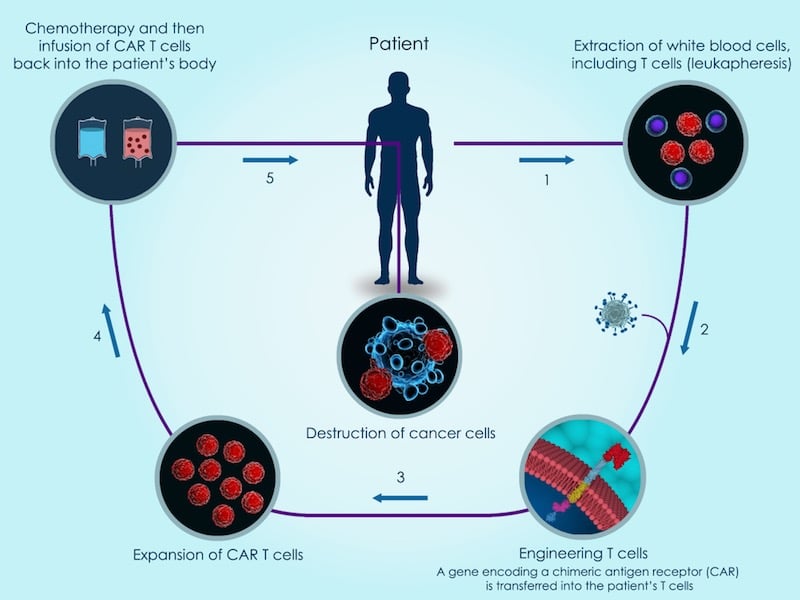
Targeting solid tumors
Currently, the only approved CAR-T therapies are indicated for rare forms of blood cancer. Extending their use to solid tumors is proving challenging because it is more difficult for CAR-T cells to infiltrate the tumor and resist the immunosuppressive microenvironment.
“How do you address solid cancers? People think ‘If you have a good target, you will hit solid cancers,” said Christian Homsy, CEO of Celyad, a CAR-T biotech based in Belgium. “No, solid cancers require a good target but also require a good T cell.”
To target solid tumors, Celyad engineers CAR-T cells to express a receptor of natural killer immune cells on their surface. The receptor, called NKG2D, can bind to 8 different proteins that are highly expressed in cancer cells and in the blood vessels that feed tumors, triggering the CAR-T cell to kill the cancer cell or blood vessels.
“The first asset we have is a very broad asset, it’s an NKG2D-based CAR which combines innate and acquired immunity,” Homsy explained. “NKG2D targets both solid and liquid tumors, so you start with a target that in itself is very exciting.”
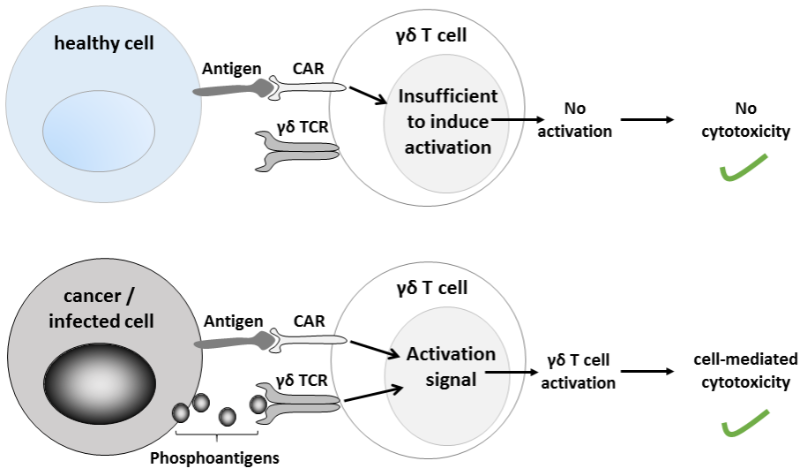
Another approach to target solid tumors gaining traction is the use of a subtype of T cells, known as gamma delta T cells, that are more effective at invading and killing solid tumors than conventional CAR-T approaches. It’s being explored by biotech companies such as TC Biopharm, GammaDelta Therapeutics or Gadeta.
Using CAR-T cells from donors
Both CAR-T cell therapies currently in the market are made using the patients’ own cells, which requires engineering them individually before reintroducing them into the patient. Celyad and other biotechs are looking at sourcing CAR-T cells from donors to make them readily available and reduce the manufacturing costs.
Cellectis, a direct competitor to Celyad, was the first to start clinical trials with an “off-the-shelf” CAR-T therapy, currently being tested in a Phase I study. Investors are undeniably excited by their technology, backing a $164M (€132M) NASDAQ follow-on offering earlier this year despite some challenges during the trial.
One of the possible complications of sourcing CAR-T cells from donors is graft versus host disease, where a patient’s immune system rejects the donor tissue. Celyad is working on reducing these types of side effects by inactivating the T cell receptors of CAR-T cells that react against the patient’s body.
“Instead of taking the T cell receptor out, we’ll interfere with the signal of the TCR inside the cell,” Homsy explained. “When you do it in animal models, you completely eradicate graft versus host disease for 60 days.”
No more chemo?
Usually, CAR-T cell therapy entails undergoing chemotherapy beforehand. However, Celyad reported earlier this year Phase I results showing its CAR-T therapy completely freed one patient of cancer for 9 months without prior treatment with chemotherapy.
“We got something that looked like a complete response in a patient without preconditioning. So what do you do with this?,” Homsy explained. “Maybe it’s an accident, but if I can repeat it two or three or four times, then it’s not an accident anymore. And if it’s not an accident, it opens up a new patient population that cannot have preconditioning.”

Giving CAR-T cell therapy the time it needs
Despite these promising results, CAR-T technology still has a long way to go. Novartis’ Kymriah, the first CAR-T cell therapy approved worldwide, has still not met its sales expectations, raising the question of whether therapies that cure diseases are sustainable from a business perspective.
Making CAR-T treatments affordable could become a decisive factor in determining the technology’s commercial success. Even though Gilead’s CAR-T cell therapy Yescarta, the second CAR-T treatment to be approved, comes at a lower cost than Kymriah in ALL, most people would consider it to be horrendously expensive at $373,000 (€316,000) a pop. Earlier this year, Kymriah was approved to treat patients with diffuse large B cell lymphoma, an indication in which Novartis was able to match Yescarta’s pricing. In the future, off-the-shelf CAR-Ts could help significantly reduce pricing. But it will take time.
“It’s not a pill. A cell therapy is something that will take time to establish itself,” said Homsy.
To watch the full interview with Christian Homsy, click here.
Images by Photographee.eu, Meletios Verras, urfin/Shutterstock