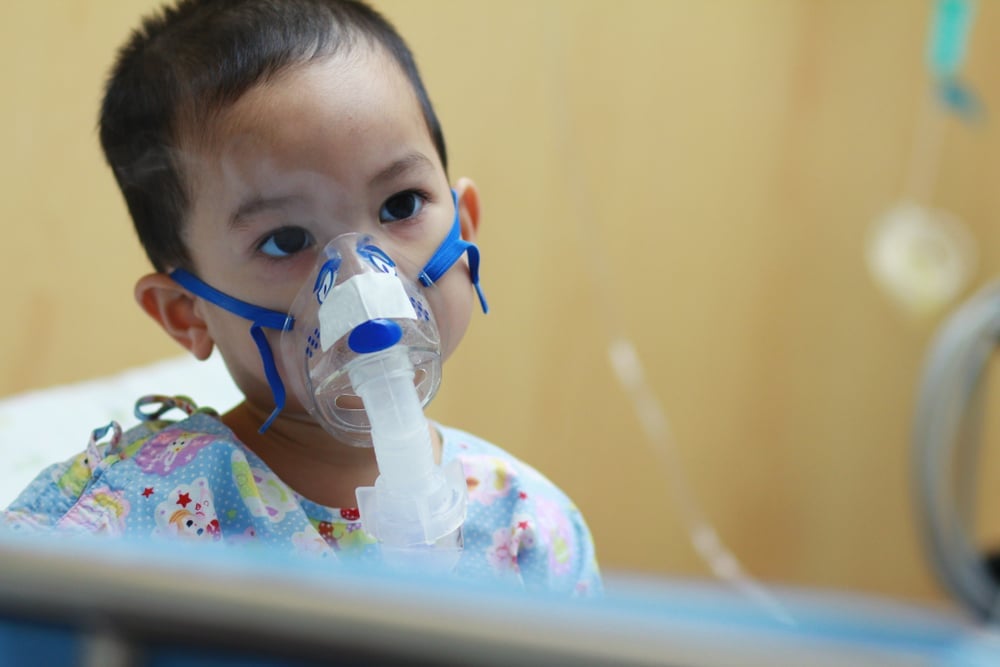
AstraZeneca and Sanofi’s Beyfortus (nirsevimab) has been approved in the European Union (EU) for the prevention of respiratory syncytial virus (RSV) lower respiratory tract disease in newborns and infants during their first RSV season.
Beyfortus is the first and only single-dose RSV passive immunization for the broad infant population, including those born healthy, at term or preterm, or with specific health conditions.
RSV is a common and highly contagious seasonal virus, infecting nearly all children by the age of two.
The European Commission is the first regulatory body to grant approval to Beyfortus.
RSV is the most common cause of LRTI, including bronchiolitis and pneumonia in infants. It is also a leading cause of hospitalization in all infants. Globally, in 2019, there were approximately 33 million cases of acute lower respiratory infections leading to more than three million hospitalizations, and it was estimated there were 26,300 in-hospital deaths of children younger than five years. RSV-related direct medical costs, globally – including hospital, outpatient and follow-up care – were estimated at €4.82 billion ($4.78 billion) in 2017.
Beyfortus
Beyfortus (nirsevimab), a long-acting antibody designed for all infants for protection against RSV disease from birth through their first RSV season with a single dose, is being developed jointly by AstraZeneca and Sanofi using AstraZeneca’s YTE technology.
Beyfortus was developed to offer newborns and infants direct RSV protection via an antibody to help prevent LRTI caused by RSV. Monoclonal antibodies do not require the activation of the immune system to help offer timely, rapid and direct protection against disease.
Beyfortus has been granted marketing authorization in the EU for the prevention of RSV LRTI disease in newborns and infants from birth during their first RSV season. The recommended dose of Beyfortus is a single intramuscular injection of 50mg for infants with body weight below 5kg and a single intramuscular injection of 100mg for infants with body weight more than 5kg.
Sanofi Alliance
In March 2017, AstraZeneca and Sanofi announced an agreement to develop and commercialize nirsevimab. Under the terms of the agreement, AstraZeneca leads all development and manufacturing activities, and Sanofi leads commercialization activities and records revenue.
Under the terms of the global agreement, Sanofi made an upfront payment of €120m, has paid a development milestone of €30m and will pay up to a further €465m upon achievement of certain development and sales-related milestones. The two companies share costs and profits.
Sobi agreement
In November 2018, AstraZeneca agreed to sell U.S. commercial rights for Synagis (palivizumab) to Swedish Orphan Biovitrum AB (publ) (Sobi) in addition to the right to participate in payments that may be received by AstraZeneca from the U.S. profits or losses for nirsevimab.
Under the agreement AstraZeneca received upfront consideration and also received non-contingent payments for nirsevimab during 2019-2021. AstraZeneca is also entitled to receive certain milestone payments for nirsevimab, including a $175m milestone following the date on which the Biologics License Application (BLA) for nirsevimab is accepted for filing by the FDA and a $90m milestone payment following the date on which BLA approval in the U.S. occurs.
AstraZeneca will continue to manufacture and supply nirsevimab globally and is entitled to an additional royalty from Sobi if profits from nirsevimab in the U.S. exceed a pre-specified level.
AstraZeneca and Sanofi answered some questions from Labiotech.eu about the news for our in-depth newsletter on children’s diseases.
When it came to finding a solution, what were the steps involved, and how difficult a challenge was it to address?
“RSV prevention has been notoriously hard to achieve. Research began more than 60 years ago and until now options have been limited to infants identified to be at highest risk of developing severe illness from the virus. This has left a number of infants unprotected. Beyfortus’ approval as the first preventative option for a broad infant population is the culmination of an eight-year clinical trial program, including three pivotal studies. Following antibody development, we added our YTE technology to extend the half-life of Beyfortus so that it can help provide protection with a single dose for an entire RSV season,” said Tonya Villafana, vice president, global franchise head, infection, AstraZeneca.
What kind of improvement in numbers will we see from this treatment?
“Globally, in 2019, there were approximately 33 million cases of acute lower respiratory infections leading to more than three million hospitalizations, and it was estimated that there were 26,300 in-hospital deaths of children younger than five years. RSV also places an enormous burden on families and healthcare systems. RSV-related direct medical costs, globally —including hospital, outpatient and follow-up care — were estimated at €4.82 billion in 2017,” said Jon Heinrichs, associate, vice president, and head of innovation and emerging sciences, Sanofi.
“Beyfortus may lead to a paradigm shift in RSV prevention as the first and only single-dose preventative option designed for the prevention of RSV lower respiratory tract disease for the broad infant population, including those born healthy, at term or preterm, or with specific health conditions. The totality of the data that we have generated indicates that protection with Beyfortus is around 80% in the all-infant population. If Beyfortus were to be approved and widely used around the world, we could potentially prevent 80% of the 33 million cases we currently see each year – a number that’s likely even higher in a RSV season as bad as the one we are in now.”
Following its approval in Europe, how will it be rolled out (and when)? And will it reach those in most need?
Sanofi and AstraZeneca said they are targeting the 2023/2024 season for a product launch and availability. As European national authorities determine their recommendations, we look forward to working with them to secure the right level of access and ensure the medical community is ready for the implementation of Beyfortus.
“We have seen the recent spikes in RSV cases around the world, including in the United States, which underscore the need for a preventative option against of RSV in infants. We are dedicated to working as quickly as we can to reduce the impact of severe RSV disease and continue to participate in discussions with healthcare authorities and are currently targeting launches in 2023,” the companies said.
Will this lead to treatments for other diseases, and are there any other disease treatments for children currently in the pipeline?
“We are pursuing broad population coverage for Beyfortus, in which we envision it being added to the immunization schedule for infants prior to or during their first RSV season. Based on the clinical findings to date, we believe Beyfortus has the potential to help alleviate the heavy RSV disease burden in newborns and infants and lead to healthcare cost reductions via a decrease in medical services and hospitalizations due to RSV disease. We are not currently investigating the use of Beyfortus in other diseases at this time.”
What are the features of RSV that make it such a problem in infants?
Louis Bont, paediatric infectious diseases specialist leader of the Utrecht RSV Research Group, University Medical Center Utrecht, said: “RSV infects virtually all children before the age of 3. Most infections are mild. However, RSV is associated with substantial mortality. More than 100.000 deaths occur annually in children younger than 5 years of age worldwide. Most deaths occur in the first 6 months of life. 97% of deaths occurred in low- and middle-income countries. (DOI: 10.1016/S0140-6736(22)00478-0).
“Pediatric wards and pediatric intensive care units are swamped with infants with RSV infection every year, during the winter season.”
According to a new study led by Bont at UMC Utrecht, one in 56 healthy term-born children in Europe is hospitalized because of RSV during the first year of life (1.8%). One per thousand of all newborns born in five European countries required intensive care unit admission to survive RSV infection.
The vast majority of RSV-related hospitalizations occur in previously healthy term-born infants. In high-risk infants (premature babies, infants with congenital heart or Down syndrome) the risk of severe disease leading to hospitalization in the first year of life is 5-10%.
Prior to Beyfortus’ introduction, what were infants treated with, and what kind of results were being seen?
Treatments for RSV diseases are mainly supportive, the companies said. Treatments include administering oxygen when patients have difficulty breathing (if necessary via intubation), and intravenous fluids or feeding. There is no specific antiviral treatment, like oseltamivir for influenza treatment.
ICU admission is required in 5-10% of infants hospitalized for RSV infection. Intensive care, in particular mechanical ventilation, prevents fatal outcomes in virtually all infants.
Prior to Beyfortus, palivizumab (another human monoclonal antibody) was approved to prevent RSV. It has been approved for high risk infants, including infants with congenital heart or preterm birth. It has not been approved for healthy term infants. Although safe and effective, palivizumab use has been limited due to its high-cost and the requirement to administer monthly doses.
Beyfortus has the advantage to require a single injection to protect all infants during their first RSV season.
What makes this so much more serious in infants?
In infants, RSV-associated lower respiratory tract infection often presents itself as acute bronchiolitis (inflammation of the small airways in the lungs). This causes mucus production in the airways. As the airways of infants are narrow, they easily run into breathing problems. Of note: airway resistance is inversely proportional to the radius, to the power of 4. Therefore, a small change in diameter has a huge effect on the resistance of an airway e.g. halving the radius of an airway would cause a 16-fold increase in resistance.
Although the biological mechanisms behind severe RSV disease are not fully understood, it is generally considered that severe RSV disease is associated with an inadequate immune response to RSV. The immune response causes an exacerbated inflammatory response and mucus production of the airways which can have negative consequences to the host including damages to the airways epithelial cells (cells located on the surface of the airway).
Infants are particularly at risk of severe disease as they encounter RSV for the first time and because of their immature immune system.