We talked to CEO Øystein Soug and CMO Magnus Jäderberg of Targovax about why the company’s technologies may succeed where others have failed.
In 2010, Targovax rose from the ashes of Norsk Hydro Pharma, the discontinued therapeutic development arm of the conglomerate. The Oslo-based biotech is taking a stand against tough-to-treat cancers like that of the pancreas with its peptide vaccine and oncolytic virus platforms.
Targovax’s peptide vaccines are based on RAS peptides that Norsk Hydro began to develop. The one that became the biotech’s lead candidate, TG01, was first used to treat a patient in 1995, but its progress stalled when the conglomerate divested from its pharmaceutical programs.
“TG01” wasn’t picked up again until the 2000s, when physicians at the University Hospital of Oslo noticed that patients who had tumors surgically removed survived for longer than historically expected after treatment with the peptide. The technology became the foundation of Targovax and received Orphan Drug Designation in both the EU and the US in 2011.
In 2015, the peptide platform was joined by a virus-based oncolytic immunotherapy one when Targovax merged with Helsinki-based Oncos Therapeutics. Following the pipeline boost, the company went public on Oslo Axess in 2016, raising 114M NOK (€14M).
We caught up with Øystein Soug, who joined Targovax as CFO in 2015 and became CEO last year, and Magnus Jäderberg, who has served as CMO since 2014, to hear about how the company is making its mark on the immuno-oncology space.
How would you respond to those who might see your vaccines as “recycling old wine”?
Soug – I think the biggest difference between the ones that have failed and our approach is the RAS target. When you look at those who have failed with vaccines and particularly also in pancreatic cancer vaccines, they have never had the type of target that we have.
Jäderberg – If you take recent examples like Newlink or Aduro, as for many previous cancer vaccines, they had to guess what kind of antigens the patients were expressing instead of a technology that will deal with a known target. At Targovax, we have a known target, RAS, for which our TG vaccines are specifically made to interact with.
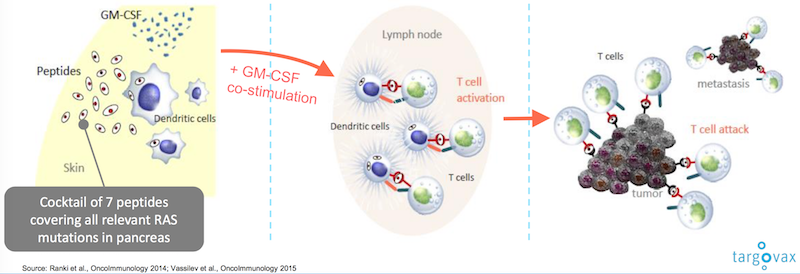
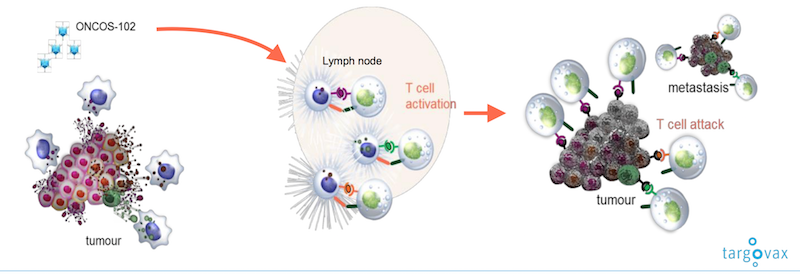
Our oncolytic virus platform doesn’t need a target though. By injecting the virus straight into the tumour, the tumour will release the specific antigens or “barcode” of each individual patient, and the immune system will then recognize these antigens as a threat, take over and kill the tumour. These oncolytic viruses are the first generation of a new way to treat solid tumours.
The viruses have quite an interesting history. About a hundred years ago, surgeons who treated patients with cancer often found that patients who had concurrent viral infections sometimes did better than those patients who just had a cancer. You would think that it’s enough for the immune system to fight one type of threat, but to have two would be more difficult — not so, it turns out.
There’s only one oncolytic virus on the market so far, Imlygic, which was launched by Amgen eighteen months ago. So it’s really the beginning of a very interesting phase, and at ASCO we saw more and more oncolytic virus research coming through the pipeline and more and more data suggesting that oncolytic viruses will be part of the future cocktail of solid tumour treatments.
So now that we’re talking about treatments versus cures, could you tell me why you chose that approach as opposed to trying to sell yourself as a cure company?
Jäderberg – It’s just our way of not exaggerating what we think is achievable. No one has yet come up with a cure for cancer. The only cancers that can be truly cured are the ones where the surgeon can cut it out completely. Maybe in five, ten or twenty years we will have a cure, but not yet.
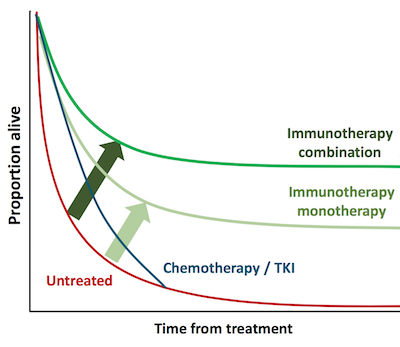
When I helped to develop Yervoy, the first checkpoint inhibitor, at Bristol-Myers Squibb, we included patients with advanced melanoma – in those patients where it was not possible for surgeons to cut out their tumours, and many of them had metastases to liver and lungs and other places. Since the Phase II trial was conducted between 2002 and 2006, there are still patients in that trial who are alive now, fifteen years later. That’s an extraordinary achievement by the checkpoint inhibitors.
But have the patients been cured? I suppose you could say, if you’re brave… I would say instead that their immune systems got the help of a checkpoint inhibitor and managed to keep the cancer at bay. That’s why we decided to use the phrase ‘manage to turn cancer into a chronic disease,’ meaning we can keep the cancer away, but we may need to repeat immune therapies like vaccinations.
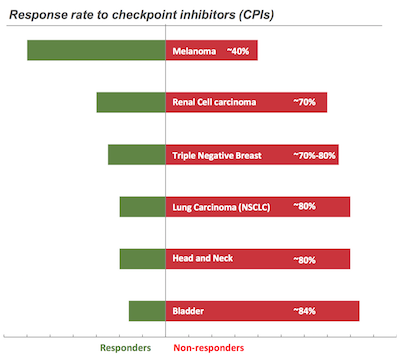
Soug – Cancer may be developing along the same path as HIV/AIDS did fifteen years ago: People died of HIV/AIDS back then, but they don’t anymore — they get a cocktail of drugs that keep them alive. They may not be 100% well, but at least they don’t die. When checkpoint inhibitors came, people thought they’d be the silver bullet that might cure cancer.
We see that although checkpoint inhibitors have tremendous effects in some patients, they don’t in most of them. Typically, apart from melanoma, the response rate is only between 15% to 30%. In most of the rest of the cases, only chemotherapy, radiotherapy or palliative care is left.
So what we are trying to achieve, with our two technologies, is to be part of the various cocktails that will be – I don’t want to use the word cure, but the drug that takes away the effects of the disease. The difference between cancer and HIV, of course, is that HIV is one disease and cancer is many different diseases – certainly in the hundreds with as many different treatment paradigms. We think that in many of these, checkpoint inhibitors in combination with other therapies such as chemotherapy and immunotherapies like our immune activators will play an important role in the future care of cancer.
Immuno-oncology is made out to be such a crowded space, but given that cancer is actually a range of different disease, do you think that the overcrowding is actually not an issue?
Jäderberg – There’s a huge number of trials going on at the moment — perhaps 800 with immuno-oncology products. What all the investors and clinicians and patients would like to know is which combinations will succeed.
At a conference 9 months ago, a panel of experts were asked which new treatment or combinations of treatments they thought would be successful when an FDA representative said, “The combination that can show extended survival will win.” So I suppose it was half of a joke, but it also speaks to the need to continue to focus on survival as an endpoint.
Also, as our knowledge of the immune system expands, particularly of immune markers in cancer, it will become easier to dissect the list of technologies and decide which particular technology is suitable for which cancer and which patient. We call this precision medicine.
It’s already happening, as Merck recently had approval for its checkpoint inhibitor Keytruda in the treatment of cancer patients based on biomarker data. Previously, we have only seen approvals in specific indications so this was a first. You can see the beginning of a trend in which biomarkers such as immune markers will become more and more important in treatment strategies.

Do you think hype is a fair way to describe the excitement about the immuno-oncology field?
Jäderberg – Hype to me would suggest that there is a financial reference to your question, and if that’s what you’re referring to, then yes, some immuno-oncology companies have been valued extremely highly like some T-cell companies that haven’t yet launched anything.
The scientific and clinical belief is that these products will have an even more important role to play in the future. Last year, immunotherapies represented about eight billion dollars in terms of sales, and the financial forecasts are that the figure will triple or quadruple in the next 8 to 10 years. So I suppose that there are a lot of people who believe that this area will expand, to the benefit of patients, but clearly also potentially to the benefit of investors.
Soug – Valuing biotech companies is like alchemy. I don’t think there are any true answers here. But hype? Of course I don’t like the word hype — it means that something is based on nothing. We think it’s based on something. People will live longer and the numbers that Magnus talked about, they will be justified by people living longer, by people not dying of cancer.
Images from blvdone, MagickStock / shutterstock.com, Targovax





