Biotech entrepreneur Darrin Disley, ex-CEO and co-founder of the successful Cambridge gene editing biotech Horizon Discovery, has a new quest — creating the perfect cell therapy. He spoke to me about his position as CEO of new biotech Mogrify and why he thinks now is a good time to get into cell therapy.
Following the meteoric rise of Horizon Discovery from a small startup in 2007 to a company of 500 people and a market cap of more than €445M, Disley decided to step down as CEO of the gene editing company in February 2018. After taking a year off to travel and “get fit and healthy” he is now back in Cambridge and firmly back on the biotech scene to head up a new cell therapy biotech.
Mogrify, which has recently raised a seed round of €3.3M to kick start its technology development, specializes in transforming one type of cell into another. The key difference from other cell transformation methods is that the company can find the chemical recipe needed to flip one adult cell into another adult cell type, without transforming it into a stem cell first.
“If you could take a cell from one part of the body and turn it into any other cell at any other stage of development for another part of the body, you effectively have the Holy Grail of regenerative medicine,” enthused Disley.
Unlike many in the biotech business, Disley was in the fortunate position of being able to take his time and pick his new project carefully.
“I need to be authentic to who I am. Going into someone else’s business that’s been going for 15 years is not really me. I’ve been offered lots of these types of opportunities, turnarounds on public markets, companies that have already done a couple of funding rounds, going into a bit of difficulty or even just doing well and just wanting to change the leadership,” he told me.
“I need to learn something new every day. I love complexity and I love taking complex problems, finding what the key issue is and finding science and technology solutions around them,” he emphasized, adding that Mogrify certainly ticked that box.
“What really inspired me is that this is a decade’s worth of science coming to fruition, not something that’s been invented in the last few months. There’s really deep science behind it.”
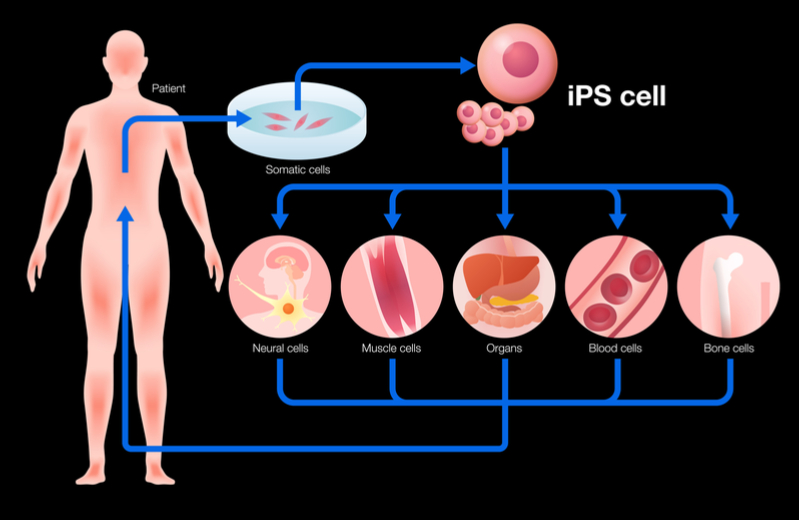
Cell transformation is currently possible, but only by using specific proteins called transcription factors to trigger a cell to go back to an immature or pluripotent state, which has the potential to become any other cell type. Scientists must then drive the pluripotent stem cell to transform into different cell types with the hopes of achieving the correct cell type needed for the therapy in question.
While the discoverers of this process were awarded a Nobel prize for their work, it is not an easy process. “For a decade, the entire world of research only managed to do around ten conversions of cells. It’s been a very slow, painstaking process,” explained Disley.
When they finally reach adulthood, a process that can take up to 15 years, the cells transformed in this way are not always completely functional in the way that a normal cell of this type would be — something that has caused headaches for developers of cell therapies. Another problem is safety, as driving cells to proliferate is also a major function of cancer.
“What Mogrify does – and this is the key bit – is it effectively bypasses all of that. It enables you to go from an adult cell type to any other adult cell type. You are not following evolutionary biology,” Disley told me.
Mogrify’s scientific founders collected genetic data on many different cell types over the last 10 years.With this information they were able to develop an algorithm that represents human cell state and can allow researchers to efficiently and accurately predict what chemical recipe is needed to switch from one adult cell type into another.
While cell therapy has a lot of potential, there are still many issues holding scientists and companies back. For example, the complex and expensive manufacturing process that is required to produce an effective cell therapy, as well as issues with getting such innovative therapies past the regulatory authorities.
I asked Disley why he chose to enter the field now. “I think it’s a good time because there is a critical mass of technologies coming together and Mogrify could be a fundamental technology in that area.”
“It parallels gene editing. When we first started [at Horizon], there was almost no one doing it and nobody saw the point. Things were just too difficult, too expensive, took too long, people didn’t see some issues as tractable,” he explained.
“That’s what’s happening in cell therapy. There’s been so much work done in so many different aspects of it that it’s all now starting to come together. People see the market’s becoming really big… Lots of biotech, lots of academic routes, lots of pharma – they are all developing the solutions to the bits and pieces that are required to come together to create a pipeline for these therapies.”
While it’s currently at an early stage, Disley believes Mogrify’s technology could help solve another issue with cell therapies — high-end pricing. He pointed out that if the company’s technology comes to fruition, it should allow the manufacturing process to be shorter, using two steps rather than five, which should be cheaper in the long run.
Disley says that at the moment the company is currently acquiring intellectual property for a range of cell transformations for different indications and in different tissue types and is also looking to set up partnerships, rather than chasing early revenue.
He says he is not the only one who seems to believe in the technology. “It’s just been off the charts. We’ve not approached anyone. People are just showering us with opportunities and they’re saying, ‘With the aid of your technologies, we can take this much further. We don’t even need to go to the pharmaceutical company.’”
Disley did not have a traditional path into biotech. He left school at 16 to try out as a professional footballer, but when this didn’t work out he got back into education in his 20’s. He says a big inspiration for him was his PhD supervisor at the University of Cambridge, Professor Christopher Lowe.
“He was effectively one of the pioneers in the UK of life sciences enterprise and here at Cambridge, too – pre-tech transfer or anything like that. At Cambridge, he was spinning companies, floating them, before there was anything here.”
In addition to his role as biotech CEO, both at Horizon and now at Mogrify, Disley has also invested in 40 different companies and regularly advises young entrepreneurs.
“I look for people who actually have a vision and it’s beyond making money,” he told me, adding that leadership potential and the ability to be flexible in difficult circumstances are also key attributes he values.
“I just look at what makes a great culture. You can’t solve the big problems of society without multidisciplinary science and interdisciplinary business models. That’s the future.”
Images via E. Resko, D. Disley and Shutterstock





