Engineered skin tissue, developed as a regenerative medicine by the UK-US pharma company Mallinckrodt, has reduced the need for skin grafts in burns victims in a phase Ib trial.
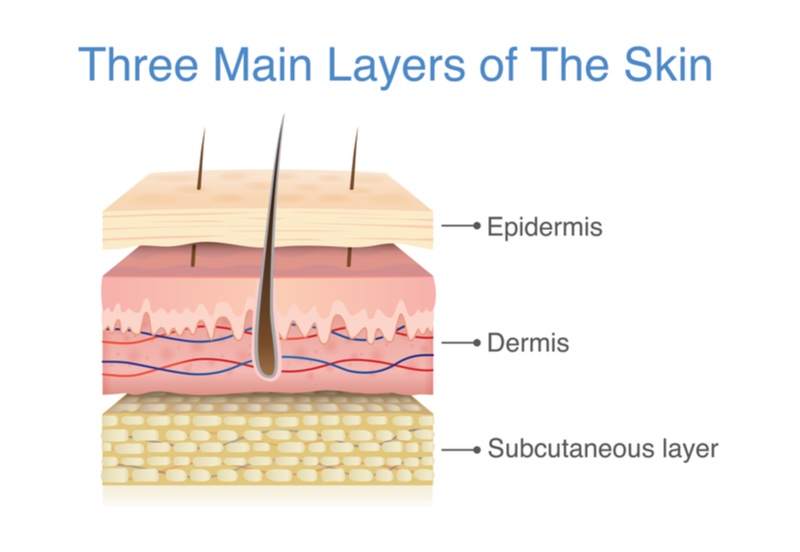
The phase I trial tested Mallinckrodt’s engineered skin treatment in 29 patients suffering from deep second degree burns. Patients with these burns have damage to the outer epidermis and inner dermis layers of the skin, and require grafts from a part of the victim’s body to help the wound heal. After one month, none of the injuries treated with the engineered skin tissue needed a graft of the patient’s skin, and 93% of the patients had their wounds fully closed three months after receiving the engineered skin.
Skin grafts from the patient’s own body are the current standard of care for patients with deep second degree burns, but can cause pain and risk infections in the area where the skin is taken from. Mallinckrodt’s off-the-shelf skin tissue is made by culturing skin cells called keratinocytes, and is designed to mimic the epidermis and inner dermal layers, reducing the need for skin grafts.
“In the last two to three decades, survival among burn patients has increased, but there have been few advances in the treatment of severe burn wounds,” stated James Holmes, Director of Wake Forest Baptist Medical Center’s Burn Center, US, and first author of the clinical study published in the journal Burns. “New approaches are needed to help minimize the challenges associated with autografting, the current standard of care.”
Mallinckrodt is also running an ongoing phase III trial of the same engineered skin treatment for second degree burns victims. If the phase III trial works out, then the company expects to apply for FDA approval next year. The FDA placed the treatment on the Regenerative Medicine Advanced Therapy list in 2017, meaning that the market approval of the treatment can be accelerated.
There is big potential for synthetic, or ‘printed’, organs like skin to regenerate the body after injury. For example, the French biotech 3d.FAB is working with the French company LabSkin Creations to develop a treatment where physicians can 3D-print skin onto wounds in the operating theater, with clinical trials expected within the next year. Another French company, Poietis, is also working on printing skin tissue to treat chronic wounds such as diabetic foot ulcers, and is currently at the preclinical stage.
Images from Shutterstock