As gene therapies become increasingly successful, companies are in a competitive race to deliver manufacturing technologies that can streamline large-scale production at reduced costs and faster turnaround times. However, plasmids, the building blocks of viral vectors, remain a bottleneck in gene therapy manufacturing. Two companies have united to provide exciting alternatives.
With over 2,400 gene therapies in the global pipeline and more than 20 gene therapies on the market today, the field is booming. Most gene therapies in the pipeline are in preclinical development and have huge potential to help patients with difficult to treat diseases.
However, the question remains: how can drug developers make sure gene therapies become available for every patient in need?
The challenge of consistency and cost

While the gene therapy field has seen huge investments pouring in, the development of large-scale manufacturing technologies is lagging behind.
“Fundamentally, we are still using a lot of the technologies that were developed 20 or 30 years ago, so the technologies that support the industry have not caught up entirely,” said Ryan Cawood, Chief Scientific Officer at OXGENE, a WuXi Advanced Therapies (WuXi ATU) company.
One main reason lies in the complexity of gene therapy development, which involves viral particles and cell lines, as well as a number of complicated production steps. The current lack of robust platform technologies means that the smallest change in production conditions can result in significant differences in the end product.
“If you’re making something relatively simple, like paracetamol, you can pretty much consistently guarantee that every time you manufacture it, you will end up with the same product,” explained Cawood. “That’s not the case in gene therapy.”
“So having a process that is scalable, where you can do the same over and over again and get exactly the same result is fundamental to being able to deliver a therapeutic that gives you the same effect every time. But the more complicated the treatment is, the more challenging that becomes.”
Another reason why there is a need for scalable manufacturing technologies for gene therapy production is the cost factor. While gene therapies remain difficult to manufacture, they will also stay extremely expensive.
The high costs for gene therapies mean that they can either only be developed for rare diseases where only a few patients need the treatment, or only for those people or healthcare providers who can afford it.
However, the aim of many companies is to one day develop gene therapies for indications with large patient populations, such as cystic fibrosis, cancer, diabetes, hemophilia, and heart disease. And for that, the manufacturing of gene therapies has to become cheaper and more scalable.
Plasmids: the building blocks and bottleneck of viral vectors
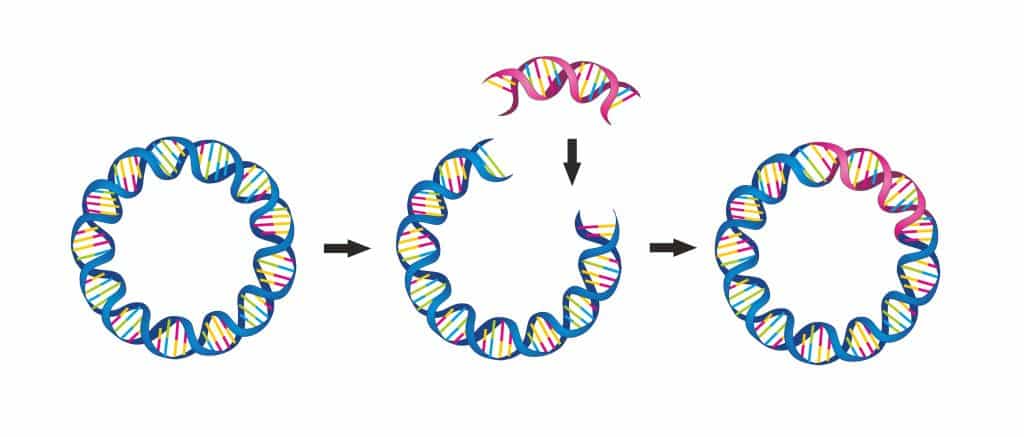
Gene therapies typically use viral vectors as vehicles to deliver the therapeutic solution – the DNA sequence encoding healthy genes – into the patient cells. Most gene therapies available on the market and in clinical development use two types of viral vectors: adeno-associated viral vectors or lentiviral vectors.
Traditionally, plasmids are used to manufacture these vectors. Plasmids are small, circular molecules of double-stranded DNA carrying the genes encoding viral vectors.
However, plasmids currently form a major bottleneck in gene therapy manufacturing.
One of the main challenges is the production of plasmids at a Good Manufacturing Practice (GMP) level, which is required by the regulatory authorities.
Establishing a GMP-grade process is complex and expensive because it requires high-quality raw materials and validated production facilities since the end product will be injected into humans.
On top of that, “the ability to manufacture GMP-grade plasmids is fairly limited globally. There are a number of providers but not as many as you might expect given how fundamental they are to the manufacture of gene therapies,” Cawood said.
Another limitation for the production of plasmids is their genetic instability, which makes them susceptible to changes in sequence, particularly when encoding complex viral vectors. The instability becomes a potential issue “when you’re scaling something from a few molecules of DNA up into the billions, possibly trillions of copies,” Cawood emphasized.
Regulatory authorities, like the FDA, also recommend the elimination of antibiotics in the production process, due to the potential risk of serious adverse reactions in patients, as well as to avoid the spread of antibiotic resistance.
This regulation causes another bottleneck for the gene therapy industry that has been using antibiotics over the last 30 to 40 years and now has to look for alternative antibiotic-free technologies.
Plasmid-free production of viral vectors
In March 2021, WuXi ATU acquired the UK-based contract research and development organization OXGENE with the main purpose of addressing the current challenges in scalable gene therapy manufacturing as a combined entity.
OXGENE works on the engineering side, improving plasmid-based manufacturing systems and engineering customized viral vectors. WuXi ATU takes a platform approach to streamline the large-scale manufacturing process of antibiotic-free plasmids and viral vectors at a GMP level.
While one part of OXGENE and WuXi ATU is working to improve the existing technology to upscale plasmid and viral vector production, the two companies have also come up with an alternative approach.
“Plasmids are what people have used for a long time, but they’re not very scalable,” said Cawood. “So, how do we remove that batch to batch variability that we see in the manufacturing process and develop new technologies which are much more scalable?”
OXGENE has developed two technologies that bypass the need for plasmids in viral vector production.
TESSA™ is an efficient, scalable way to manufacture adeno-associated viral vectors. Cawood explained: “TESSA™ uses a modified adenoviral vector to deliver all of the components of adeno-associated viral vectors, so you no longer require plasmids.”
XLenti™ packaging and producer cell lines, on the other hand, are used to manufacture lentiviral vectors.
“We’ve integrated the pieces of DNA needed to manufacture lentiviral vectors into the cells themselves. We grow these cells up, we send a trigger to the cells to start producing lentiviral vectors, and then they produce the lentiviral particles for us. So we don’t have to introduce new plasmids because all of that DNA is already inside the cell”, described Cawood.
To ensure the continued safety and high quality of the company’s manufactured products, WuXi ATU’s testing division tests the products continuously along the manufacturing pipeline. This also helps prevent delays at the final stages of gene therapy manufacturing, where the smallest mistake can set a therapy’s development back several months or even years.
Towards a better future: getting large-scale manufacturing right
Together, OXGENE and WuXi ATU can support gene therapy developers from start to finish – from the moment they have the idea for a new gene therapy to the very end of a successful product, the large-scale market release.
The two companies are continuously working to optimize large-scale gene therapy production. WuXi ATU, for example, is currently adjusting its facilities to be able to manufacture TESSA™ vectors, and recombinant adeno-associated viral vectors using TESSA™ at GMP level.
“In the future, we will see many alternative technologies being adopted as the landscape for manufacturing gene therapies changes. Adopting technologies like TESSA™ will allow people to use the existing footprints that we already have, but to be able to manufacture more gene therapies within those footprints,” Cawood concluded.
Curious to learn more about GMP-grade plasmid production or alternative approaches to viral vector manufacturing? Get in touch with the team at OXGENE and WuXi ATU or visit their websites for more information!
Images via Shutterstock.com and OXGENE





