News and Trends 29 Jun 2023
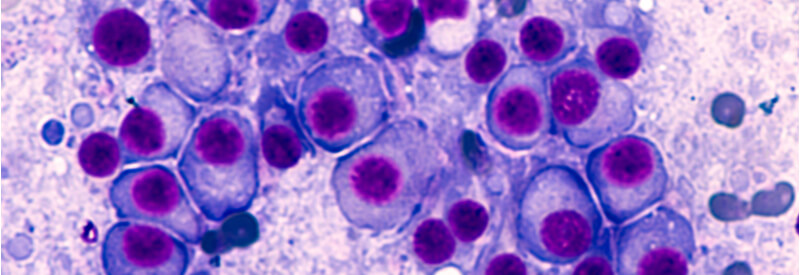
FDA gives Orphan Drug Designation to CellCentric multiple myeloma drug
CellCentric, a UK-based biotechnology company, has announced that the U.S. Food and Drug Administration (FDA) has granted orphan drug designation for inobrodib in the treatment of multiple myeloma. Delivered as an oral capsule, inobrodib can be used at home without requiring intensive monitoring. It is a first-in-class drug, with a new mechanism of action. It […]