News and Trends 16 May 2023
SiSaf siRNA therapy gets FDA designations to treat autosomal dominant osteopetrosis
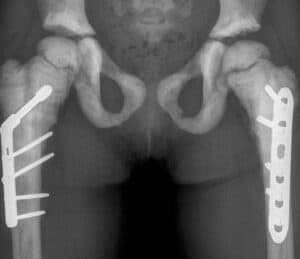
SiSaf Ltd, an RNA delivery and therapeutics company, has announced that SIS-101-ADO, its siRNA therapeutic for patients with autosomal dominant osteopetrosis Type 2 (ADO2), has been granted orphan drug designation by the U.S. FDA. Also, due to the serious manifestations of this rare skeletal disorder in children, SIS-101-ADO has been granted rare pediatric disease designation […]