News and Trends 5 Sep 2022
Avacta’s drug targeting soft tissue sarcoma granted Orphan Drug Designation by FDA
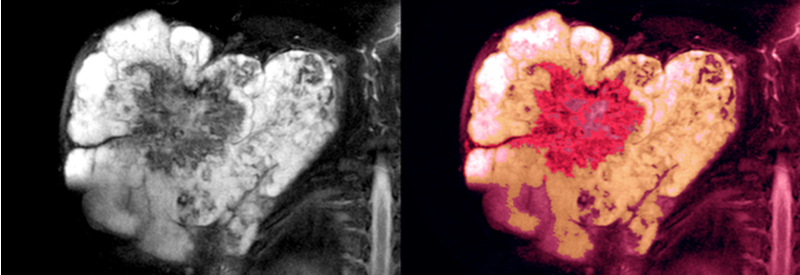
A drug to treat soft tissue sarcoma has been granted Orphan Drug Designation (ODD) by the U.S. Food and Drug Administration (FDA). Clinical stage oncology drug company Avacta Group plc has produced a form of the generic chemotherapy, doxorubicin AVA6000. About Avacta’s drug The drug has been modified using the company’s pre|CISION technology and Affirmer […]