A Migraine is more than just a headache; it is a neurological disorder affecting a staggering 10% of the worldwide population. So where are we on fighting a viable therapy – and who is working on Migraines?
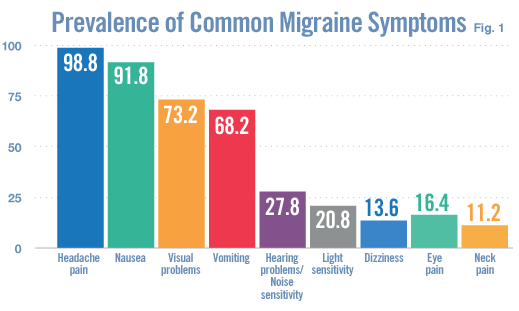
People of all ages are at risk of migraine, with women being three times more likely to suffer from the condition than men. The throbbing head pain is frequently accompanied by nausea, vomiting, and sensitivity to light and sounds.
The attacks can last from a few hours to several days and occur on a daily basis, rendering the sufferers completely debilitated, caused by a wide variety of environmental, chemical and hormonal factors.
Current treatments include pain-killers, vasoconstrictors, Opioids, Glucocorticoids, Beta-blockers, antidepressants, anti-seizure drugs and even Botox injections to the face and neck (to numb the nerves). However, most of these are only effective for relatively mild attacks, have severe side effects – and can even be addictive.
Fortunately, a breakthrough discovery in migraine research may deliver the long overdue relief. Migraine attacks were found to be accompanied by increased blood levels of a neuropeptide called calcitonin gene-related peptide (CGRP) implicating the peptide in migraine pathogenesis.
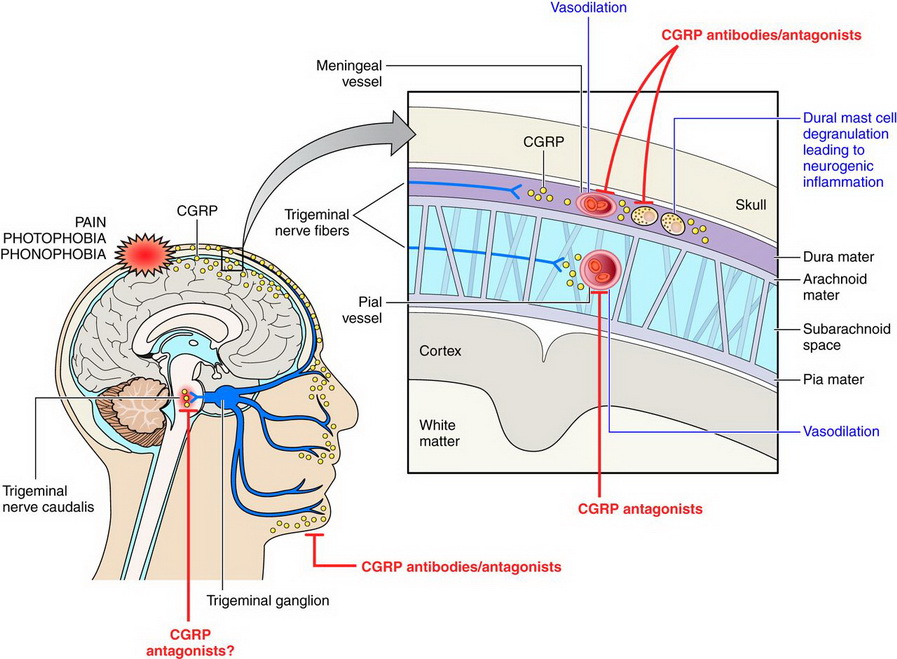
This neurotransmitter was found to act as a vasodilator of the brain’s blood vessels when it is released from the trigeminal nerves, which are responsible for pain in the face and head.
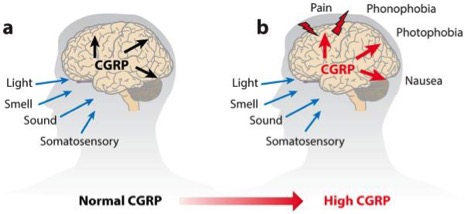
Studies have even shown that elevated levels can forecast migraine episodes, and when injected into the blood, CGRP caused a migraine-like headache within hours in migraine-pronged patients. On the other hand, since ‘healthy’ patients only developed a mild headache, the study concluded that migraine sufferers could have a heightened sensitivity to CGRP.
The promising findings spurred the race for development of CGRP antagonists for migraine treatment. In 2000, Boehringer Ingelheim designed a small molecule called bibn4096bs, which was effective in stopping migraine attacks in some patients, but had adverse side effects.
Then Merck (Germany) also followed suit with its own small compound, but the trial was pulled in 2011 due to unacceptable levels of toxicity to the liver.
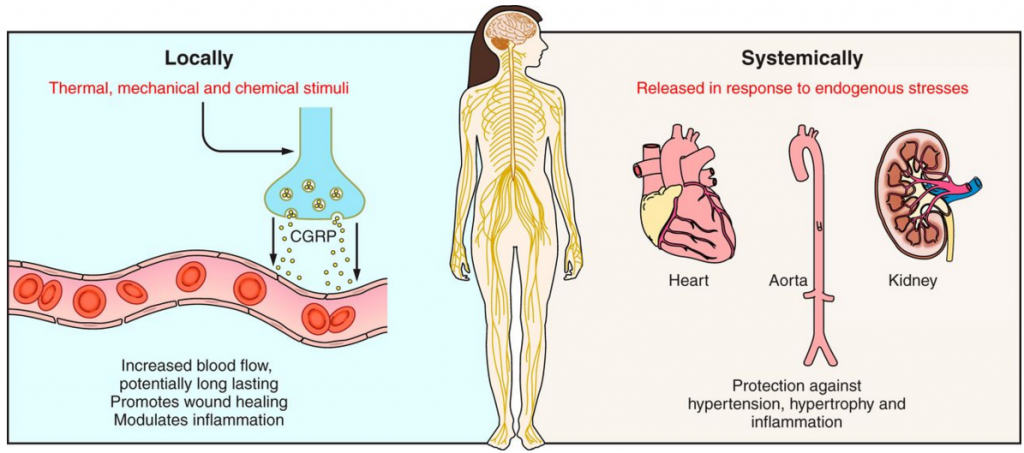
Since none of the studies have safety and efficacy data beyond a six month period, and because CGRPs are widely distributed throughout the body, prolonged use could potentially have adverse effects on the brain, lungs, heart and kidney function…
This was the lesson learned from Merck, and the multifunctional roles of CGRP in the body meant ensuring the specificity of CGRP antagonists is essential. Thus, the second generation of drugs comprised the CGRP peptide-blocking monoclonal antibodies.
Merck cut its losses and sold the rights for two novel CGRP antagonists to Allergan (Ireland) in 2014 for $250M.
Allergan (having since been acquired by Pfizer for €140Bn) is now expected to launch a phase III trial this year on one of the antagonists, MK-1602, for acute migraine treatment, and a phase II trial on the second drug, MK-8031, for migraine prevention.
And only 4 players are in the race for approval of the first migraine antibody drug. These include Teva (Israel) with the drug called TEV-48125 as well the US based Amgen, Eli Lilly and Alder Pharmaceuticals. In addition, Teva has partnered up with the Cambridge biotech Heptares Therapeutics (UK) for their platform which targets CGRP related G protein-coupled receptors (GPCRs).
All four achieved favourable results in Phase II trials, demonstrating migraine prevention in two thirds of the patients, with up to 15% of patients experiencing complete relief. However, despite this overwhelming success, researchers want to exercise cautious optimism…
So migraine sufferers will unfortunately have to wait for these phase III results before labelling such antibody drugs as the ‘Holy Grail‘ for migraines.





