The FDA puts one of Adaptimmune‘s TCR trials on a partial hold, pending more information about the production of its cell therapy. It’s more waves for the Biotech’s financial rocking boat.
![]() Oxford-based Adaptimmune is one of the European players in TCR therapies. Besides being listed on the NASDAQ, it got GSK on board on a €311M deal to develop its leading program, TCR NY-ESO. These therapies target a peptide associated with multiple cancers.
Oxford-based Adaptimmune is one of the European players in TCR therapies. Besides being listed on the NASDAQ, it got GSK on board on a €311M deal to develop its leading program, TCR NY-ESO. These therapies target a peptide associated with multiple cancers.
This program is now suffering a hiccup, as the FDA has put one of its trials on a partial hold. The hold affects a Phase I/II trial for myxoid round cell liposarcoma (MRCLS), a rare form of cancer. This trial isn’t active yet, nor it had recruited any patients.
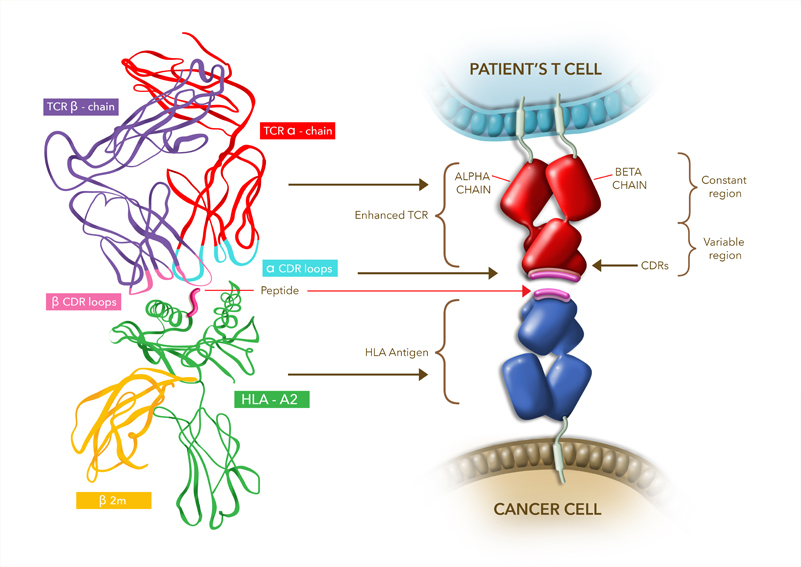
The problem doesn’t seem to be safety concerns about the therapy itself. The FDA requested additional information about the therapy’s production (chemistry, manufacturing and controls) and about the design of the clinical trial. Adaptimmune stated that it’ll answer the FDA shortly, but has given no estimation of by how much this could delay the trial.
Furthermore, the therapy is already in several other clinical trials. Adaptimmune is currently conducting 5 other Phase I/II trials for different cancers (synovial sarcoma, multiple myeloma, ovarian cancer, melanoma and non-small cell lung cancer).
So it seems that the clinical development of cell therapies is still not straightforward, even with Adaptimmune’s background. It may be that the FDA is now being stricter with the field, especially after the death of patients in a CAR-T trial from the company Juno.
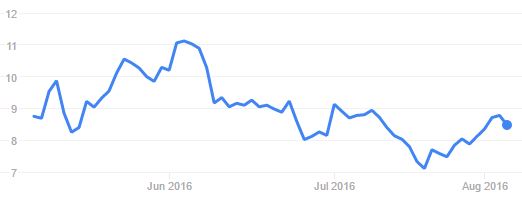
While this isn’t a big setback for Adaptimmune, FDA holds aren’t great news either. Since the announcement, the Biotech’s volatile stock valuation is again on a downward trend.
Despite solid results from its technology and entering clinical development, Adaptimmune has faced hard times on the NASDAQ. Its share price has gone from $17 (at the time of its IPO) to the $7-$11 range in the last months.
UPDATE (Original Publication 05/08/2016): The hold has just been lifted by the FDA, reports FierceBiotech, as Adaptimmune has met its demands for more “information regarding the move from an academically derived to a commercially viable manufacturing process.” Shares have since risen by 14%, on top of the 16% gain this week.
Feature Image Credit: Pixabay





