Anergis (Switzerland) has gotten €4.5M from investors and is planning a phase IIb trial for ATIBAR, its leading candidate to treat Tree allergies.
![]() Anergis is developing ultra-fast immunotherapies for allergies, which could be an interesting alternative to current treatments, known as ‘desensitization’ or conventional Allergy Immunotherapy (AIT).
Anergis is developing ultra-fast immunotherapies for allergies, which could be an interesting alternative to current treatments, known as ‘desensitization’ or conventional Allergy Immunotherapy (AIT).
This process of inducing tolerance to the allergen requires 3-5 years – time that could be saved with Anergis’ products.
Following the completion of phase II trials, Anergis is advancing it leading programme, AllerT for birch allergies. A phase IIb trial is in the making, that will access efficacy and tolerability of two dosing regimens of AllerT In Adults with Birch Pollen AllergicRhinitis/ Rhinoconjunctivitis (ATIBAR).
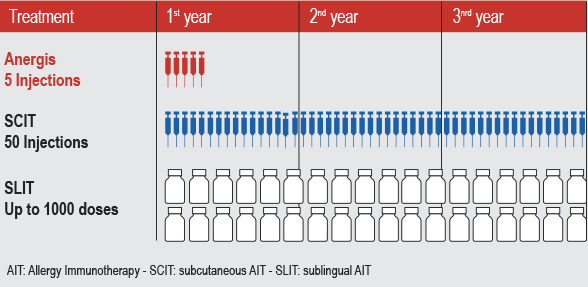
ATIBAR is expected to start in the Fall of 2016, with a total of 450 patients. Results are expected in the third quarter of 2017.
This trial is designed meet both European and US FDA efficacy criteria for allergy immunotherapy products. After this, one more confirmatory efficacy phase III trial will be required before registration, expects Vincent Charlon, CEO of Anergis.
Meanwhile, Anergis has closed a financing round extension with existing investors, which was worth around €4.5M (CHF 5M). Along with previous fundraisers, this should prepare the company for the first registration of AllerT.

Anergis’ products are based on its proprietary platform of Contiguous Overlapping Peptides (COPs), which consist of long synthetic peptides, reproducing fragments of a selected allergen (foreign protein that triggers the allergic response).
AllerT is also in pre-clinical development for ragweed and dust mite allergies.
The allergy space has seen some recent successes. DBV Technologies from Paris (France) is developing a skin patch for food allergy, and it have been very successful lastly with now a market cap over €1Bn. Another Biotech is Circassia (UK), which has an extensive portfolio in allergies and asthma – and led the biggest Biotech IPO in 2014, raising €267M.
Allergies are the most prevalent and fastest growing chronic conditions in the industrialized world affecting over 500 million people, so new therapies for allergies have the potential to tap into a big market.





