Newsletter Signup - Under Article / In Page
"*" indicates required fields
Update (17/11/2017): Motif Bio will raise up to $20M (€17M) in the form of a loan from US firm Hercules Capital. The funds will be used to fund the pre-commercialization stage of iclaprim, a new antibiotic that is expected to be launched in the US in 2019.
Originally published on 04/10/2017
Iclaprim is a new antibiotic developed by Motif Bio, a UK-based biotech, which has met the primary endpoint in a Phase III study.
Motif Bio develops new antibiotics, and its candidate, iclaprim, has met its primary efficacy endpoint and demonstrated good tolerability. This news has caused a 33% rise in the company’s share price. Having completed two Phase III trials, Motif is now aiming to submit an NDA to the FDA in early 2018.
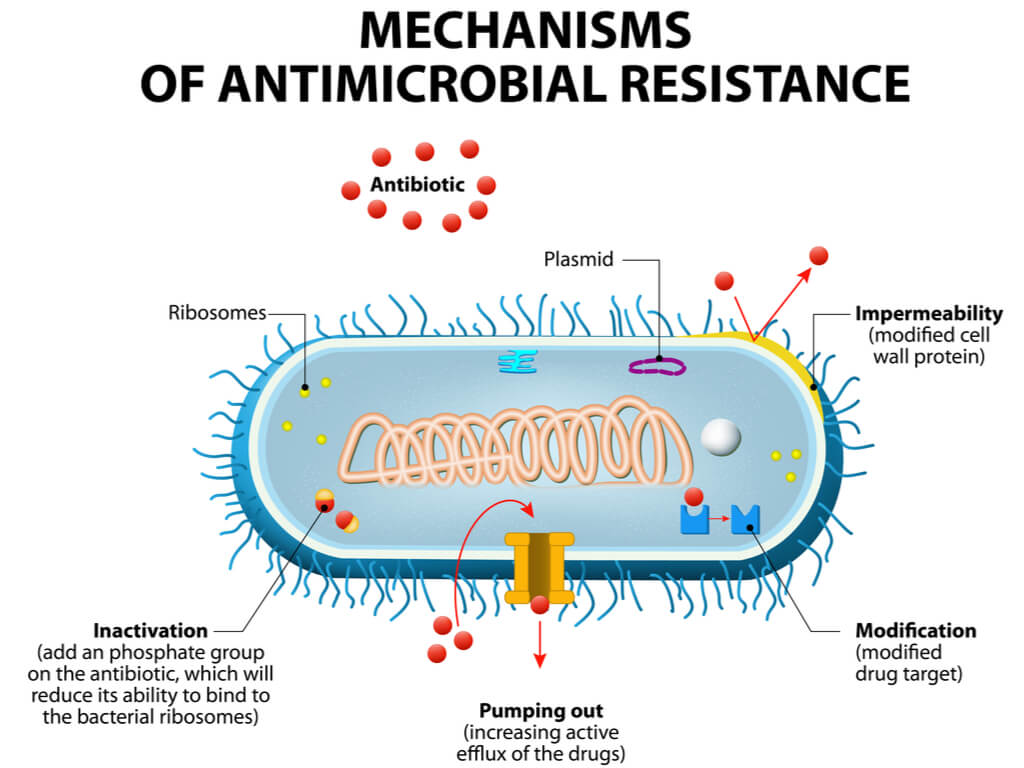
Iclaprim is a potent Gram-positive bacteria killer, which inhibits an essential bacterial enzyme, dihydrofolate reductase (DHFR). DHFR catalyses the production of tetrahydrofolate, a form of folic acid, which bacteria require to produce DNA and RNA. This underutilized approach could help the drug to escape antibiotic resistance, which currently plagues the field.
So far, iclaprim has been tested in over 1,300 patients and volunteers. In the most recent Phase III clinical trial, REVIVE-2, it was tested in patients with acute bacterial skin and skin structure infections. Iclaprim was compared with vancomycin, and demonstrated non-inferiority (<10% difference) for both the primary endpoint, early clinical response, and secondary endpoint, clinical cure.
In an effort to overcome antibiotic resistance, some big names in biotech have switched their attention to the area, including Immunocore, which received €33M from the Bill & Melinda Gates Foundation to develop its TCR technology against tuberculosis. Da Volterra got €20M from the EU to speed up the development of its medical device that prevents Clostridium difficile infections. Back in the lab, British researchers are harnessing the power of bacterial weapons for new therapeutics.
A big advantage of iclaprim is that it is administered as a fixed, intravenous dose. This has been proposed to save time, resources and costs for healthcare professionals. Another is its improved tolerability in comparison with current antibiotics, which are toxic to the kidney.
The fear is of course that bacteria will find a way to develop resistance to this new approach. They’ve done it before, so why not again. What is certain is that we need to try new approaches to this problem, and that is exactly what Motif has done here.
Images – Kateryna Kon / shutterstock.com; Designua / shutterstock.com