After Vienna, it’s time to jump in the middle of the Paris-Amsterdam-Frankfurt triangle in the BioPark of Brussels South in Belgium.
We will meet a company who can answer the problem of antibiotic use in the bacterial strains selection whilst, at the same time, increasing bioproduction yield. Our Biotech of the week, Delphi Genetics.

City: Gosselies (Belgium)
Founded: 2001
Employees: 15
Financial Data: Secured financing totaling €3.4M for its new development
Activities: Technology development and Bioproduction of Plasmid DNA, Recombinant proteins and Antibodies

Mission: Delphi Genetics is committed to developing an alternative way to select and stabilize bioproduction of products using E. coli. This is made possible thanks to its Staby® technology.
The most commonly used antibiotics in biotech processing aim to remove plasmid-free hosts from a bacterial culture. Thus, the antibiotic selection is performed since many years to increase production reproducibility.
However, those Antibiotics are expensive and represent a potential contamination risk in the fermentation process. Moreover, the presence of antibiotic-resistance genes in the bacteria represents a metabolic burden for these bacteria reducing the yield of the product of interest. Finally, the use of antibiotics for manufacturing need to demonstrate that the final product is “antibiotic-free”. The assessment of the residual antibiotic levels and their removal are costly procedures required by regulatory agencies.
The solution of Delphi Genetics is a ‘poison-antidote system’, a highly effective stabilization system based on the use of selection modules naturally found in plasmids, bacterial chromosomes and bacteriophages.
A selection module (here the ccd system) is formed out of an operon and two
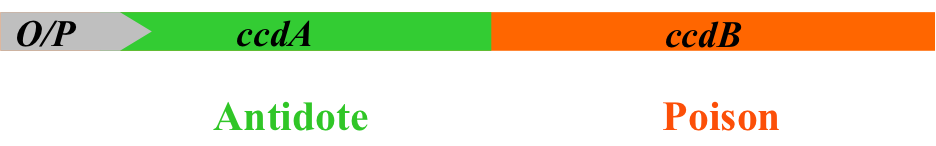
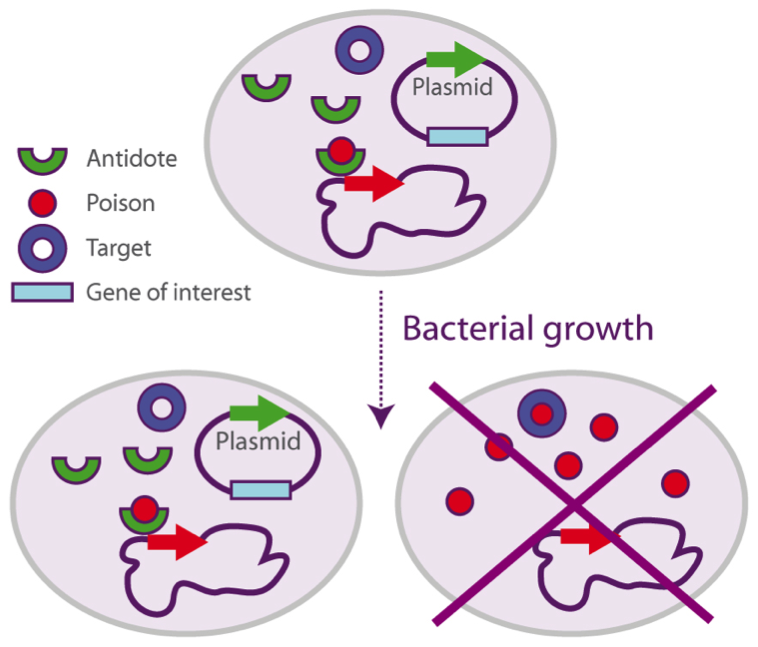
In the system, both genes have been separated: the antidote is integrated in the plasmid, whilst the poison is present in the host’s chromosome.
If the plasmid (containing the gene of interest) is not in the cell, only the poison will be expressed and therefore will induce the cell’s death. Once the plasmid is integrated, the antidote will be expressed as well, and the cell will be able to produce the proteins.
This patented technology presents some key advantages:
- An antibiotic free production process, therefore meeting regulatory guidelines.
- A scalable fermentation process. The stabilization technology is based solely on genetic elements, and is thus readily scalable (in any culture medium). The process is then easily transferred from R&D to industrial scale fermentation.
- Higher yields of expressed DNA or proteins due to perfect plasmid stabilization (control on the batch to batch reproducibility) and limited energy consumption compared to the antibiotics selection system.
Furthermore, the Staby® technology is available through customized services (strain adaptation, productions) and/or licensing. Indeed, it was validated and licensed by several big pharma companies as Sanofi-Pasteur, GSK, Merck-MSD,…
Comment:
To assist you in your pDNA development, Delphi Genetics has recently announced the investment in a fully equipped GMP production line in Gosselies. The investment has already been made and it is now expected to be fully operational within the coming six months .
The high yield production can fit your R&D research as well as the preclinical and soon the clinical trial batches, High Quality production being already available using a robust RNAse-free process.

And as innovation never really stops for Delphi, the company is also applying its technologies for the production of recombinant proteins and antibody development.
If you need more information for your project or if you are just curious to know more about this technology, perhaps you could say hello?
Visit Delphi’s website or have a chat with the team
Feature Image Credit: Labiotech Map