Axon Neuroscience is starting a Phase II trial with its immunotherapy for Alzheimer’s disease, which redirects the immune system against defective Tau proteins, thought to be the main cause of this major disease.
 Alzheimer’s disease is the cause of up to 70% of dementia cases, according to WHO, which translates in over 30 million patients worldwide. As the world population ages, Alzheimer’s is quickly becoming a major global health concern.
Alzheimer’s disease is the cause of up to 70% of dementia cases, according to WHO, which translates in over 30 million patients worldwide. As the world population ages, Alzheimer’s is quickly becoming a major global health concern.
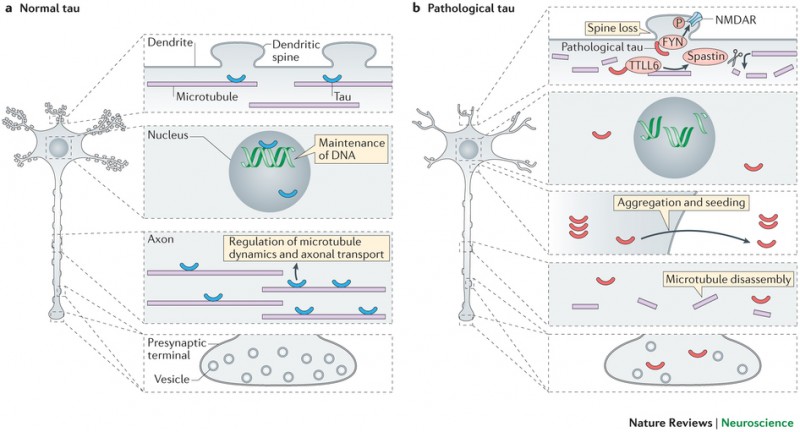
While there’s still a lot of research going on to understand the exact root causes of Alzheimer’s, defective Tau proteins seem to be a definite hallmark of this and other neurodegenerative diseases (tauopathies).
One of the biotechs targeting Tau proteins is Axon Neuroscience (Slovakia), which already has a successful Phase I trial under its belt.
Now, Axon Neuroscience is taking its AADvac1 vaccine to Phase II trials. The 2-year trial will take place in Europe, enrolling 185 patients with mild Alzheimer’s disease.
Besides confirming the vaccine’s good safety profile, the goal is to see if AADvac1 can slow down or halt the cognitive decline typical of Alzheimer’s.
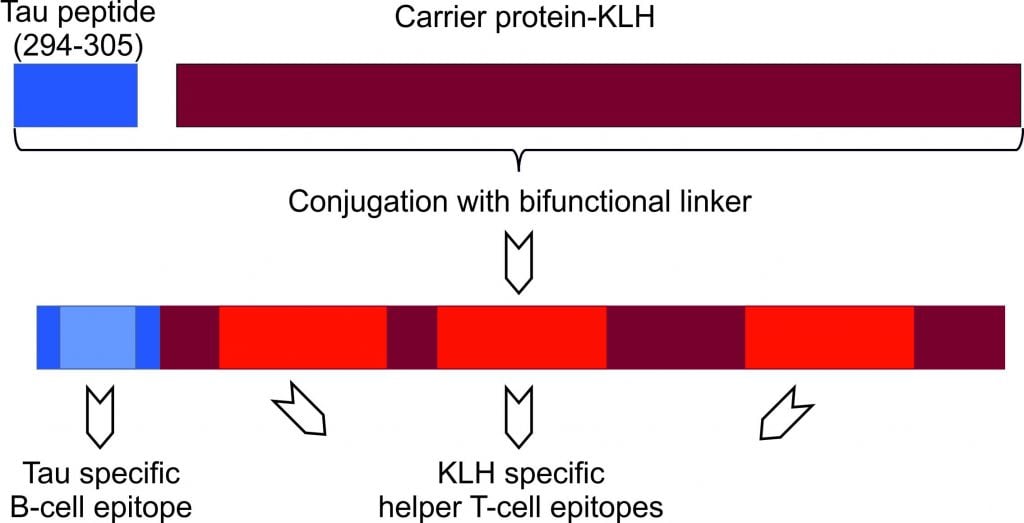
AADvac1 is an active immunotherapy, designed to direct antibodies against the pathological Tau protein. As antibodies bind to these defective proteins, it is expected that they don’t have other interactions, such as aggregating in tangles.
Tau proteins are a popular target for Alzheimer’s therapies, as we’ve covered in our review 2015’s highlights in nervous system developments. Since then, TauRx (Singapore and Scotland) has even received €120M for its Phase III trial with inhibitors of Tau aggregation.
Feature Image Credit: Neurons & Glia (CC 2.0 NICHD)
Figure 1 Credit: Wang and Mandelkow (2016) Tau in physiology and pathology. Nature Reviews Neuroscience (doi: 10.1038/nrn.2015.1)
Figure 2 Credit: Kontsekova (2014) First-in-man tau vaccine targeting structural determinants essential for pathological tau–tau interaction reduces tau oligomerisation and neurofibrillary degeneration in an Alzheimer’s disease model. Alzheimer’s Research & Therapy (doi: 10.1186/alzrt278)