Balmes Transplantation has raised €700,000 in seed funding to run a proof-of-concept study to test its drug combination for kidney damage.
Balmes Transplantation is a young biotech in Marseille, France, that develops combination therapies for a common complication associated with kidney transplants and heart surgery. The biotech has raised a €700,000 seed funding from top company executives and angel investors. This and the €900,000 that the company had already raised will support an in vivo proof-of-concept study.
Balmes targets kidney ischemia-reperfusion injury, a complication that affects up to 500,000 heart surgery patients and around 45,000 kidney transplant patients in the US and Europe each year. A temporary impairment of blood flow to the kidneys causes the death of renal cells, which can lead to kidney failure.
The biotech repurposes and combines existing drugs to work against a number of aspects of ischemia-reperfusion injuries. The planned proof-of-concept study will allow the biotech to test its first drug combination in two indications: cardiac surgery-associated acute kidney injury and delayed graft function after kidney transplants. Patrick Berna, Balmes’ CEO, told me that the biotech had decided to focus on kidney injuries as “[reperfusion injury] is also a big issue in the kidney… within transplantation, the kidney is the most transplanted organ, making up around two-thirds of all solid organ transplants.”
Balmes found potential compounds by screening the World, European and US drug databases. Those that could preserve kidney cells were combined and gave rise to some synergistic effects. The company now has 5 combinations each consisting of 2 repurposed drugs. Berna could not divulge too much about the combinations but he did say that the compounds came from quite unrelated indications: “some compounds were in hematology, some were in hypertension, others were anti-fungal… very diverse.”
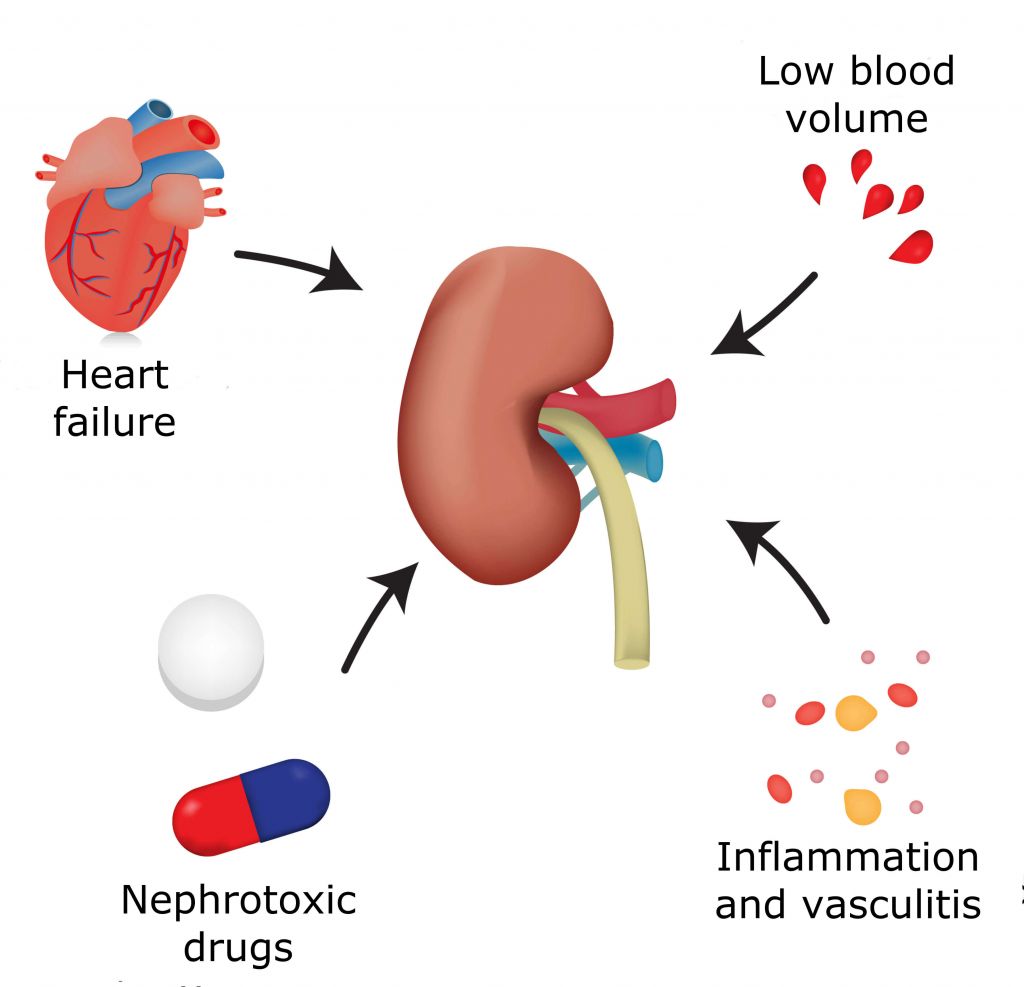
With the rates of heart surgery and kidney transplants still high, preventative measures for complications like kidney ischemia-reperfusion injury are required. British biotech Rexgenero has developed a candidate for a similar condition, critical limb ischemia, which has just started Phase III trials. Perhaps the approaches of Rexgenero and Balmes could provide inspiration for more biotechs to enter the field.
As the restoration of blood flow to the kidneys following ischemia causes cell death, regenerative medicine could also be an important area of research going forward. Scientists at Queen Mary University of London have started generating the adrenal glands using cells in urine, so perhaps this technique could be applied to the adjoining kidneys.
The repurposing of approved drugs has its advantages, including a potentially shorter route to the market, and as a result, Balmes hopes to begin Phase II trials as early as 2020. The company will kick off its proof-of-concept study in rats, and when I asked about the company’s future plans, Berna told me: “the next step will be to go into large mammals with real transplantation.”
“It would be very much of interest to test these compounds on other types of cells like cardiomyocytes or neurons,” added Berna. Therefore, if the biotech can succeed in stopping kidney reperfusion injuries, it may look at developing its combinations for the prevention of damage to the heart following myocardial infarction and the brain after a stroke.
Images – Ben Schonewille / shutterstock.com; joshya / shutterstock.com