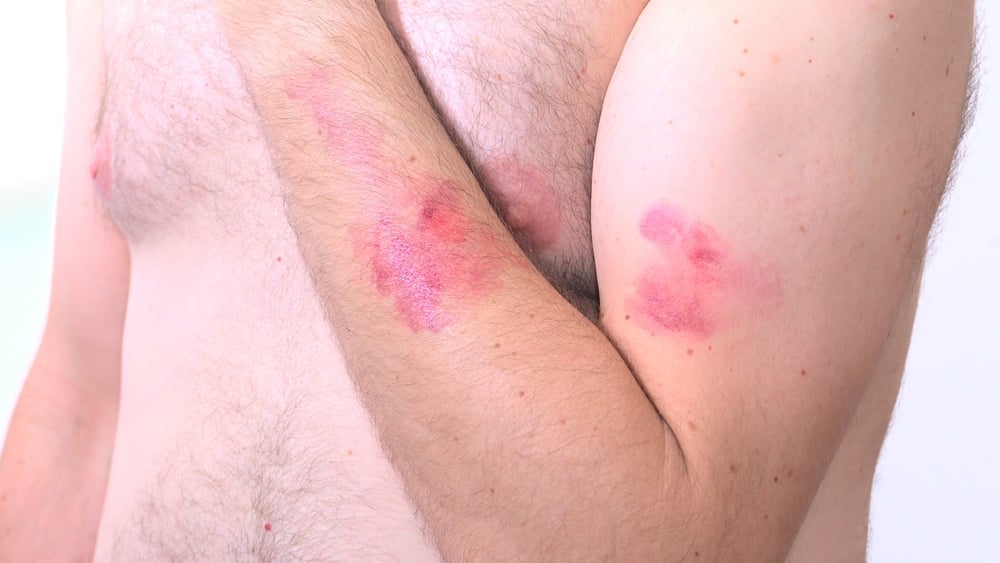
BD (Becton, Dickinson and Company), and CerTest Biotec have announced emergency use authorization (EUA) from the U.S. Food and Drug Administration (FDA) for a molecular polymerase chain reaction (PCR) assay for mpox virus detection.
The VIASURE Monkeypox Virus Real Time PCR Reagents for BD MAX System is now available for BD MAX System users.
“The mpox outbreak continues to be designated as a global health emergency — the World Health Organization’s highest level of alert,” said Nikos Pavlidis, vice president of Molecular Diagnostics at Becton, Dickinson and Company.
“The large installed base of the BD MAX System in hospital labs provides broad access to testing for a wide range of infectious diseases, now including the mpox virus. EUA for the assay enables it to be used for timely diagnosis and may help avert further global spread of the disease.”
The BD MAX System is a fully integrated, automated platform that performs nucleic acid extraction and real-time PCR providing results for up to 24 samples across multiple syndromes in less than three hours. Becton, Dickinson and Company offers an extensive menu of tests on the system covering health care associated infections, respiratory infections, sexually transmitted infections, gastrointestinal infections and women’s health.
“We were able to quickly develop the VIASURE mpox molecular test by leveraging the BD MAX System’s open system reagent suite,” said Nelson Fernandes, managing director of CerTest Biotec.
“The EUA for the assay enables use for clinical diagnosis of the disease, in addition to surveillance and the research and development of vaccines and treatments.”
As with all CerTest assays, the VIASURE Monkeypox Virus PCR Detection assay for the BD MAX System is offered in a lyophilized format. Accordingly, the assay will come in a tube that snaps into the test-specific position on the BD MAX ExK TNA extraction strip, which is supplied by Becton, Dickinson and Company.