Cellectis researchers have managed to come up with a new CAR architecture with a small-molecule triggered ‘on-switch’, permitting better control and safety in CAR T-cell therapy, as published in Nature.
 As we discussed during our interview with Andre Choulika, CEO of Cellectis, last month, one major drawback to CAR-T treatment for cancer is the lack of control one can have in how the CAR-T cells are deployed, taken up and act on cancerous cells.
As we discussed during our interview with Andre Choulika, CEO of Cellectis, last month, one major drawback to CAR-T treatment for cancer is the lack of control one can have in how the CAR-T cells are deployed, taken up and act on cancerous cells.
Simply put, there’s a lot of fine-tweaking and R&D to be done before CAR-T can truly be considered ‘the miracle cure for cancer‘. Choulika also described how engineering CAR’s is also designed to enhance drug potency, the doses of which are limited in the number of cells they can target.
Therefore, a CAR-T treatment is designed to work as a drug-conjugate treatment, as CAR’s also have a limited ‘life-span’ within the body, and have to work as efficiently as possible.
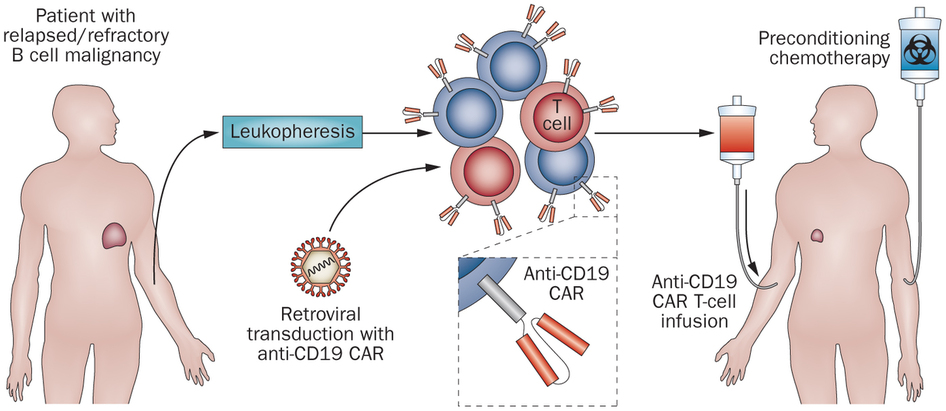
The possibility to spatially and temporally control CAR-T activity is very desirable to reduce the risk of unwanted (and often dangerous) side effects, such as cytokine-release syndrome (CRS).
To date, few safety strategies addressing this problem exist, meaning researchers have to depend on suicide mechanisms (ultimately, a complete eradication of the engineered T-cells) to stop patient tissue damage, which prematurely ends the treatment. And this is certainly a hurdle in treating the patient’s cancer!
So what did the Paper Find?
Alexandre Juillerat and team engineered a system directly integrated within the CAR structure. You can read more about how CAR’s are engineered and developed into a T-cell attack system in our review.
In particular, they demonstrated a system which can turn CAR T-cells from an ‘off-state’ to an ‘on-state’ by adding a small molecule (in this case rapamycin), which interacts with the CAR to cause a structural change.
Juillerat and team transfected primary T-cells with mRNAs encoding each chain of the multichain CAR (mcCAR), and adding rapamycin meant there was a change in the level of detection of the ‘hinge’ domain of the CAR-T.
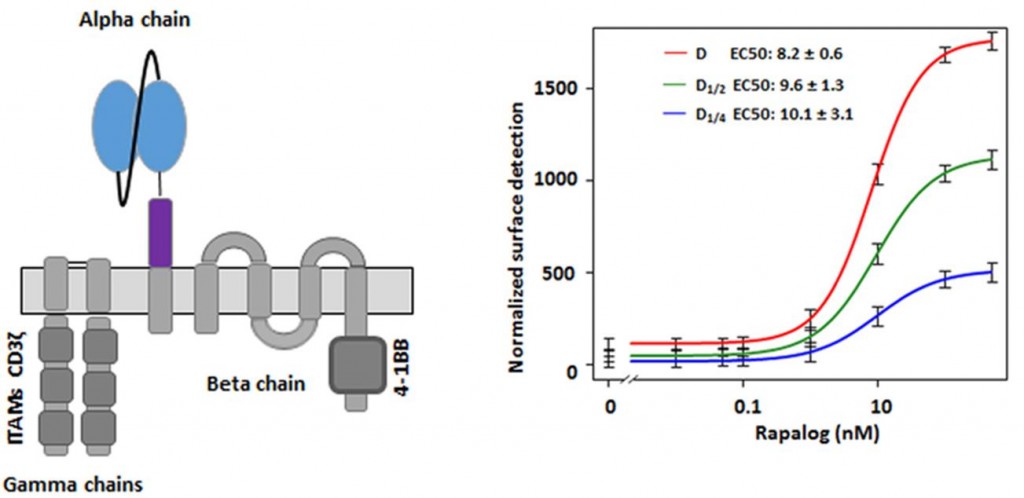
This was ‘tracked’ by rest of the CAR-T – and the section which targets the CD19 receptor (a common cancer target – and the focus of the UCART19 trials by Cellectis), and in fact dramatically improved the surface detection of the CAR-T (for some regions, up to 15-fold).
Overall, this new system would allow the CAR-Ts to be preserved (i.e. not sacrificing them for the sake of safety), as well as offering a temporal control on their action, to reduce risk of CAR-induced toxicities. The CAR-T dose could then be re-activated using a locally targeted drug – although clearly more investigation is still needed.
However, implementing non-lethal, spatio-temporal control of gene edited CAR T-cells still represents a major advancement in improving the CAR T-cell technology.
Feature Image Credit: Killing Cancer Cells (© Cellectis)