Celyad failed to get significant results in its Phase III trial in cardiac disease. However, this cell therapy could still have a positive effect in a part of the patients. True potential or public relations patch?
 Cardiovascular diseases are the leading cause of death worldwide. Biotech solutions in the field range from gene therapy (with uniQure and BMS) to enzyme stabilization (a Swedish strategy that closed a €100M deal) and even neurostimulation.
Cardiovascular diseases are the leading cause of death worldwide. Biotech solutions in the field range from gene therapy (with uniQure and BMS) to enzyme stabilization (a Swedish strategy that closed a €100M deal) and even neurostimulation.
Cell therapy also had a place in cardiovascular diseases with Celyad’s C-Cure. Celyad (Belgium) has a platform to derive cardiopoietic cells from a patient’s bone marrow. After this procedure, the cells were reinjected into the patient’s heart and differentiate into normal heart cells.
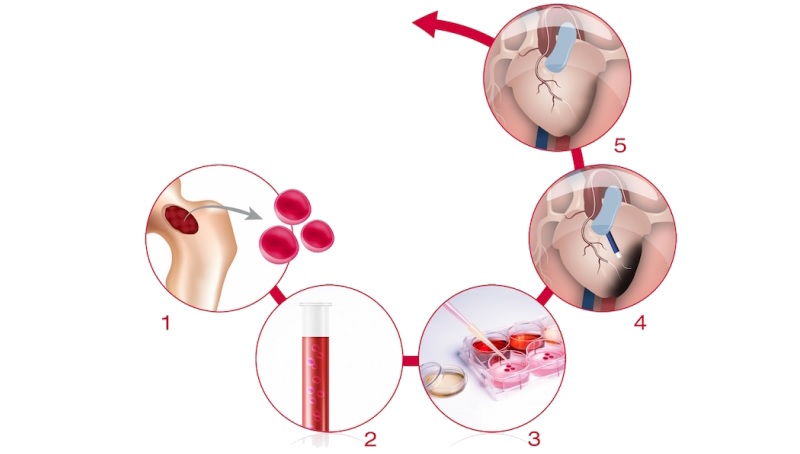
Now, topline results of C-Cure’s Phase III trial (CHART-1) were just published, but they don’t look great. The therapy didn’t meet the trial’s primary endpoint: a statistically significant improvement of outcome for patients with chronic advanced ischemic heart failure. This condition affects the blood flow and can lead to heart attack.
Lack of significant results can be particularly problematic for cell therapies. Because of their high price, the reimbursement can be harder if the clinical data are not significant enough.
However, there may still be hope for C-Cure. Celyad reports that a positive trend was clearly identifiable, and the effect of the therapy was significant in a subset of the trial’s patients.
This subset was segmented by End Diastolic Volume (EDV), the volume of blood in a ventricle just before the heart contracts (systole). This subset represents 60% of people in the study (144 out of 240).
These are only topline results of the study, and the details will be communicated at the ESC (European Society of Cardiology) in August in a “late breaking” presentation.
Based on these positive parts of the results, Celyad plans to go forward with an application for EMA‘s marketing approval. C-Cure would target the same type of patients that have shown significant improvement and for whom current treatment options are limited.
There are also plans for a second Phase III trial in the US, for which Celyad is now seeking partnerships.
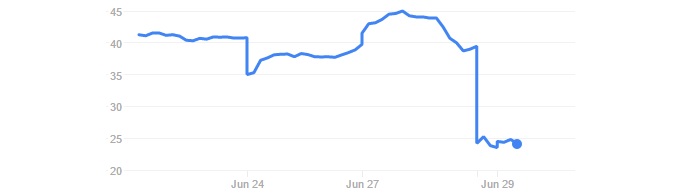
The announcement made Celyad’s stock fell by 40% on the Euronext Brussels and 31% on the NASDAQ. Clearly, the news spooked investors.
However, Celyad has other assets, namely its promising program in CAR-T (which was discussed during Refresh). While it’s still in Phase I trials, Celyad had already reshuffled its organisation to focus on oncology.
Celyad holds key patents on the CAR-T field and has a unique expertise from conducting a Phase III in cell therapy (one of the most advanced in Europe so far). The CAR-T program was also one of the main reasons why Celyad successfully IPOed on the NASDAQ and raised over $100M.
All in all, this news is obviously a bad news for the company, but it’s not yet time to jump ship.
Celyad’s CAR-T Fireside Chat at Refresh…
Feature Image Credit: Pixabay





