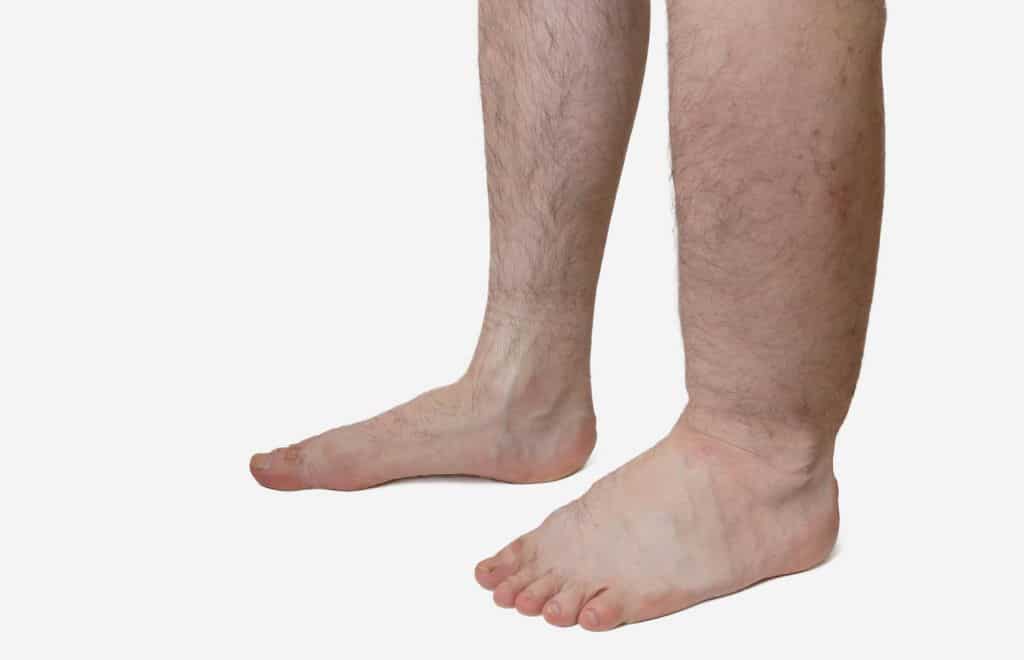
Hereditary angioedema (HAE), a condition that causes recurrent attacks of severe swelling, could be prevented by a monoclonal antibody after positive top line phase 3 results were reported.
Garadacimab, biotech company CSL’s investigational monoclonal antibody inhibiting factor XIIa, is being developed as a long-term preventive treatment for patients with HAE.
Efficacy objectives
The study met its primary and secondary efficacy objectives and also demonstrated favorable safety and tolerability. CSL aims to begin filing with global health authorities at the end of the current fiscal year for full approval.
The multicenter, double-blind, randomized, placebo-controlled, parallel-arm study (known as VANGUARD) evaluated the efficacy and safety of monthly subcutaneous garadacimab administration in the prevention of HAE attacks compared to the placebo for six months. Full results from the study will be presented at an upcoming scientific congress and published in a peer-reviewed journal.
Bill Mezzanotte, chief medical officer for CSL said: “These results underscore our belief that garadacimab has the potential to become a transformative first-in-class therapy for people living with HAE, a patient group that CSL has been serving for many years.
Disruptive innovation
“CSL’s promise to patients guides us to meet their unmet need by pursuing the type of disruptive innovation we believe garadacimab represents. We look forward to sharing the full results of our phase 3 study in the coming months.”
HAE is rare, genetic and potentially life-threatening and causes painful, debilitating and unpredictable episodes of swelling of the abdomen, larynx, face and extremities, among other areas of the body.
Garadacimab is a novel Factor XIIa-inhibitory monoclonal antibody (FXIIa mAb) currently in phase 3 clinical development as a new type of once-monthly subcutaneous prophylactic treatment for attacks related to HAE, a form of bradykinin-mediated angioedema.
Garadacimab uniquely inhibits the plasma protein, FXIIa. When FXIIa is activated, it initiates the cascade of events leading to edema formation. By targeting FXIIa, garadacimab inhibits the HAE cascade at its origin as compared with other HAE therapies that target downstream mediators.
Clinical programs
Garadacimab was discovered and optimized by scientists at CSL’s Bio21–based research site, with formulation and manufacturing for the clinical programs completed at the CSL Broadmeadows Biotech Manufacturing Facility.
Orphan-drug designation for garadacimab as an investigational therapy for hereditary angioedema has been granted by both the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA).
CSL is also investigating garadacimab for other indications, beyond HAE, where FXIIa inhibition may play an important role in improving clinical outcomes, including pulmonary fibrosis.