Galapagos will receive a $10M (€8.5M) milestone payment from its collaborator, AbbVie, after their cystic fibrosis candidate started a Phase I trial.
Galapagos is a clinical-stage biotech developing small molecule medicines for a range of indications. It partnered up with the global pharma company, AbbVie, back in 2013, to develop a number of cystic fibrosis (CF) candidates for a combination therapy. Galapagos has pocketed $10M (€8.5M) from AbbVie on the back of the news that its CF corrector, GLPG3221, will enter Phase I to test its safety, tolerability and pharmacokinetics in healthy patients.
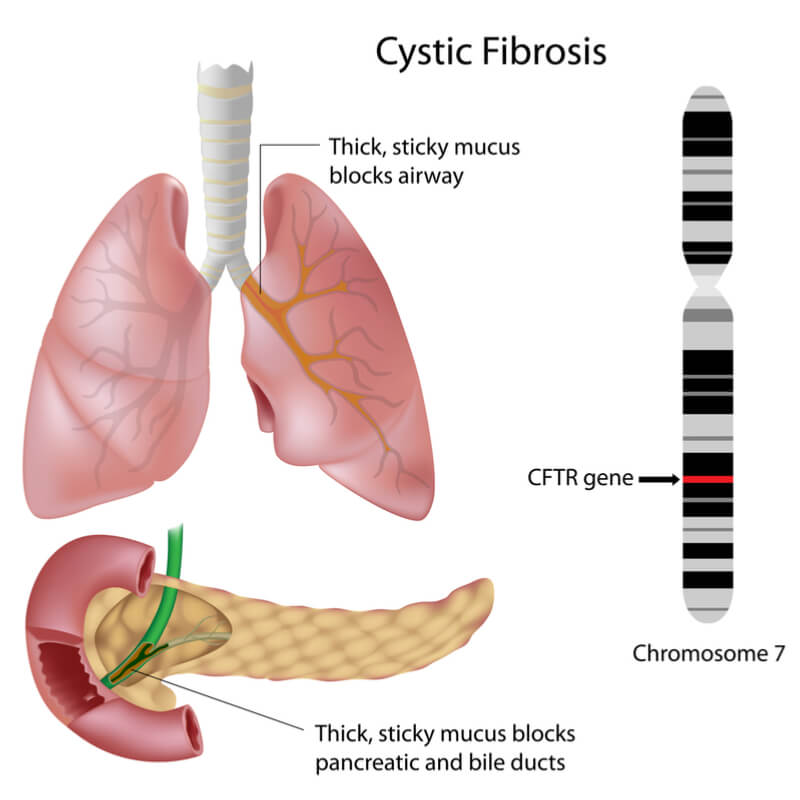
Around 70,000 people are living with CF worldwide, a progressive disease that sees patients suffer from persistent lung infections and breathing difficulties. The disease is caused by a mutation in the cystic fibrosis transmembrane conductance regulator (CFTR) gene that leads to the buildup of thick, sticky mucus in the lungs, pancreas and other organs.
GLPG3221 is one of a number of candidates that Galapagos and AbbVie are looking at to form a triple combination therapy. This particular candidate is a corrector drug, meaning it helps to restore the regular function of the CFTR channel. It is a C2 corrector so part of the second generation of these drugs, which are chemically distinct from their predecessors.
Abbvie walked away from a massive deal with Galapagos in 2015 but decided to stay on board for the cystic fibrosis project. The biotech-pharma power couple seems to have got over its initial squabbles, with this being its second Phase I trial of the year. Galapagos CSO, Piet Wigerinck, told us why the company can attract powerful partners like AbbVie and Gilead: “We just have a very attractive model for pharma.”
The global cystic fibrosis therapy market was estimated to be worth $3.5B (€3B) in 2016, but this is expected to rise to almost $14B (€12B) by 2025 due to increasing prevalence and rising treatment rate. Like Galapagos’ candidate, Vertex’s combination drug could help CF-patients to live ‘normal’ lives again. Despite its financial trouble, ProQR has completed a Phase Ib trial with its lead CF candidate, while Antabio received €7.6M to develop a new antibiotic against Pseudomonas infections.
Galapagos and AbbVie appear to be progressing nicely towards their goal of a triple combination CF treatment, with candidates trickling through to the clinic. Galapagos has received $67M (€57M) in milestone payments from the partnership so far, which has no doubt played a big part in giving its CF candidates, and the rest of its pipeline, a big boost.
Images – studiostoks / shutterstock.com; Alila Medical Media / shutterstock.com





