Given the rates of diabetes reaching ‘epidemic proportions’, we figured it was time to have a better overview of biotech’s progress in the field. With some serious advances having been made in the treatment of the disease, we have to ask – how much further can biotech go in the treatment of diabetes?
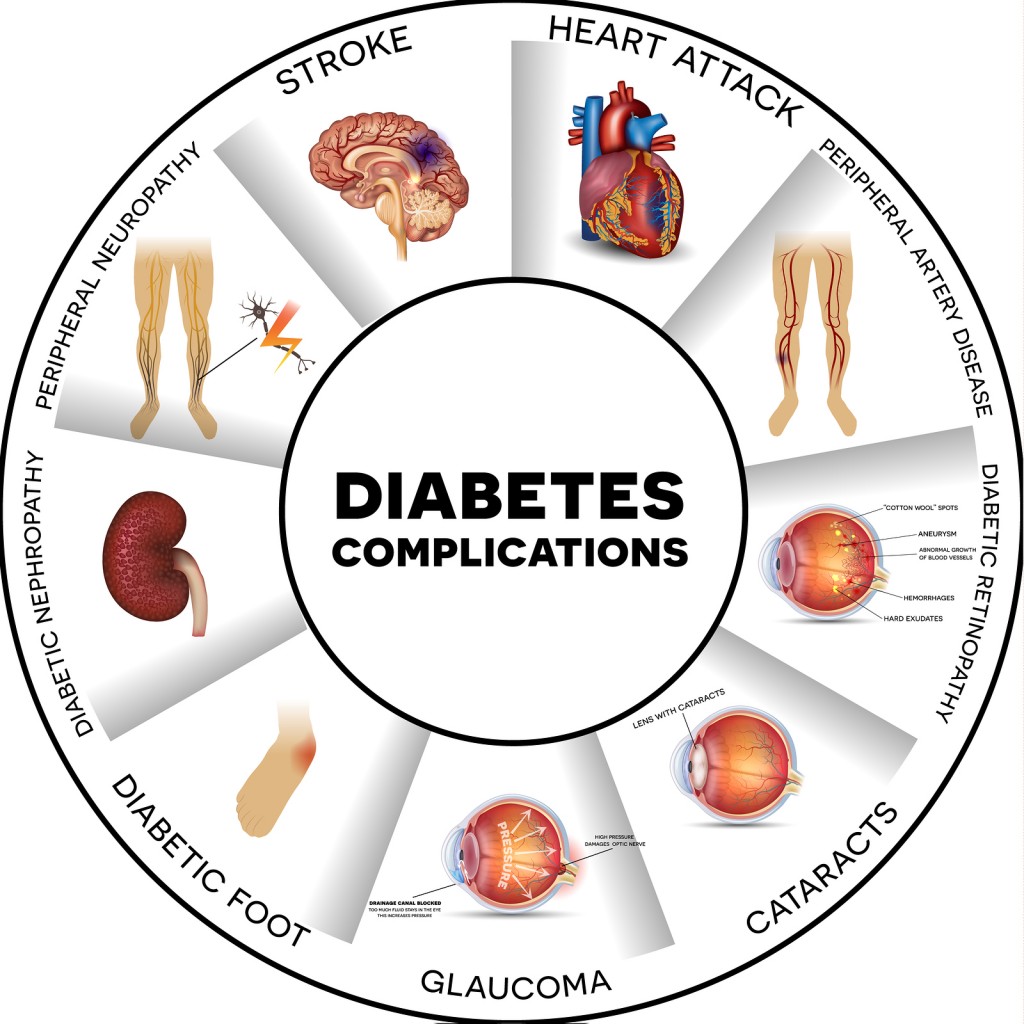
It is important to note the various complications that can arise from the disease, which has a series of serious co-morbidities. These can range from increased risk to infection, cardiovascular disease and even neuropathies (nerve damage across multiple tissues), each of which presents their own challenges to tackle.
So here’s a review of recent developments, including developments in insulin, the growing MedTech field and how cell therapy is also tackling the disease.
Improving Efficacy of Insulin
Insulin was the first human recombinant protein to be approved on the market under the brand Humulin. It was initially developed by Genentech and marketed by Eli Lilly in 1982. This was a massive step forward to replace pig insulin and improve the life of diabetics worldwide. Since then, the recombinant insulin never stopped being improved.
Enhancing existing insulin treatments and metformin have been the first line-of-treatments in tackling Diabetes (type I and type II respectively).
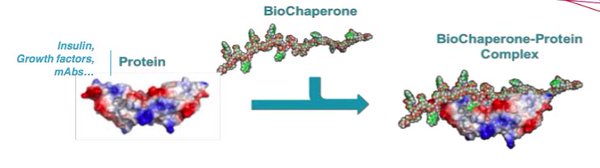
Although treatments vary between types, generally improving synthetic insulin is done by either increasing the absorption rate of synthetic insulin, new formulations (analogs – e.g. Lantus) or introducing biotechnological enhancers, such as BioChaperones. One example of the latter is the French biotech Adocia’s Biochaperne Lispro, which went on to be licensed by Lilly in 2014.
Additionally, other treatments include once-daily human analogs of the naturally occurring hormone Glucagon-Like Peptide-1 (GLP-1), which only stimulates the release of insulin when glucose levels become too high.
Many are working on these GLP-1 analogs as a treatment for type 2 diabetes – from Novo Nordisk’s Diabetes Care Unit to Sanofi (partnered with Denmark’s Zealand Pharma) and GSK etc.
The general principle here though is to combine these therapies with insulin analogs to maximize the effect. However, one of the main challenges in diabetes care is a sustainable and reliable drug delivery.
MedTech: The Solution to Needle-Free Digitisation of Diabetes?
A big emphasis in the industry has been to find needle-free methods for delivery, given the obvious discomfort and hassle the repeated use of needles can cause (compliance, mistake of medication).
Improving Delivery
Over the last decade, an increasing number of drugs, from hormonal contraception to anti-smoking aids, have become available as transdermal drug delivery systems (TDDS). These are non-invasive patches that facilitate drug absorption through the skin.
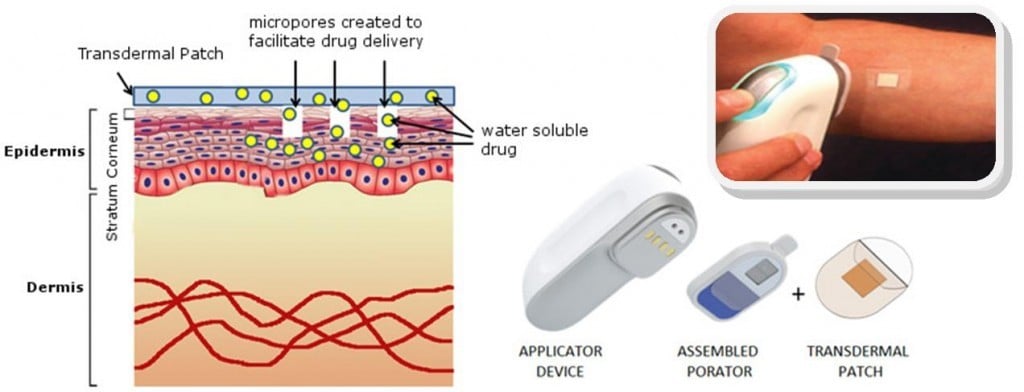
For insulin delivery, one example is Prometheon Pharma, which has developed the TruePatch, a multi-day basal insulin patch. Patches can also be problematic and irritating (e.g. cosmetically).
So another approach is the Intarcia implant from Boston (the Medici Drug Delivery System), partnered with the French pharma Servier. This is a futuristic matchstick-sized intra-dermal pump that has undergone three Phase III trials in conjunction with a GLP-1 agonist (exenatide).
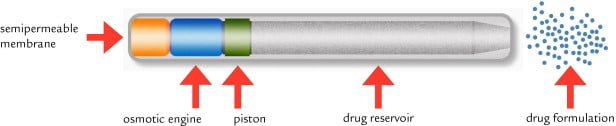
Ultimately, if approved, the ITCA 650 system (pump and GLP-1 formulation) would represent the first injection-free therapy capable of delivering up to a full year of treatment from a single device. Regulatory filing in the US is projected for later this year.
Replacing the Lancet
There’s also a whole range of monitoring tech out there designed to avoid the use of lancets to test glucose levels. These include GlucoSense (a spin-out company jointly formed and funded by NetScientific and the University of Leeds) that uses laser technology and NovioSense (a Dutch start-up in Nijmegen), which is a 15 mm-long metal coil that uses tear fluid to measure glucose.

On the other hand, Gluco-Wise is a new product from MediWise (in London), which is also an optical reading device designed for the ear-lobe. There is also the hotly discussed Google Lens being developed in conjunction with Novartis. Trials are due to start for the lens this year (read more via IEEE Spectrum), however, it’s not so clear whether this is a lot of ‘hot air’, as one ex-Verily employee leaked.
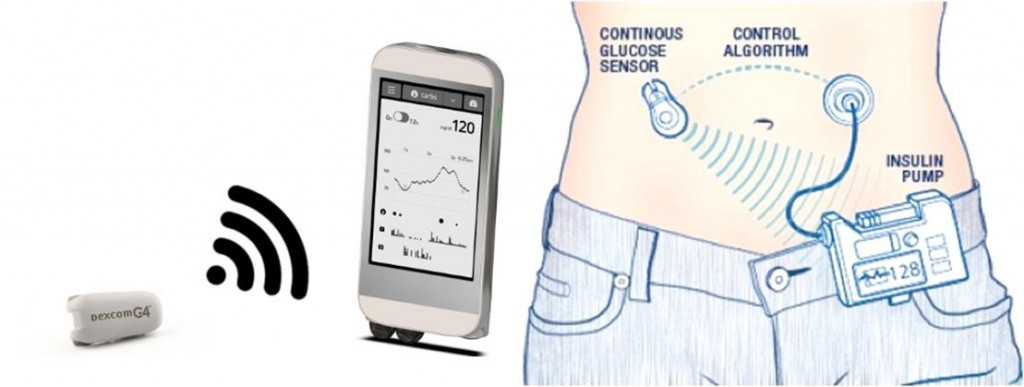
Roche is also making progress in its Diabetes Care Unit. It has struck a deal with the MedTech company Senseonics to sell an implantable glucose sensor in some major European countries.
With an app, patients then can access real-time glucose measurements. The software also predicts the ‘wobble’ in glucose levels, so that patients can take steps to stay in control if they are near to developing a hypo or hyperglycemic episode.
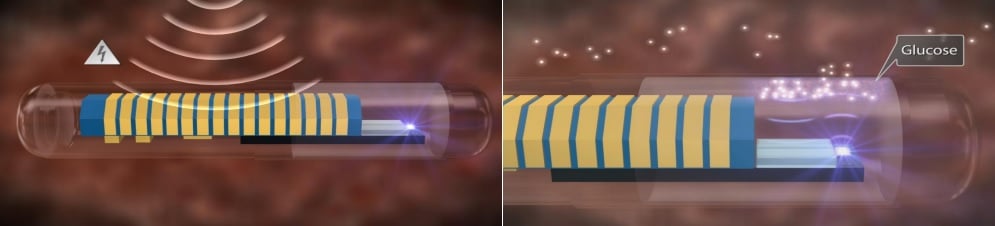
A Monitoring-Delivery Combo?
Automated pancreatic simulation is an interesting alternative to the standard insulin injections, as these might not always best match the rapid changes in blood glucose throughout the day. In addition to an intradermal pump, Cellnovo’s mobile system comprises a handset from which the patient can control the pump.
A research team from the University of Cambridge has also managed to develop a closed loop beta-cell based insulin delivery system for diabetes type I. This bionic pancreas prototype has been constructed for human trials using an iPhone app which monitors blood glucose levels.
A similar research development has been made by Boston’s Bionic Pancreas project, which completed its 22-day outpatient trial in April of last year. In collaboration with Boston University and Massachusetts General Hospital, the Bionic pancreas has been branded the iLet.
However, this kind of system has been scrutinized by the ‘Biohacking’ community and even parents – particularly in the States, where this kind of MedTech could potentially be too far-off from regulatory approval or too costly for some patients.
But is Cell Therapy the Ultimate Cure?
Bolstering up dysfunctional tissue with transplant tissue to enhance insulin production is also a popular area of development.
The Diabetes Cell Therapy Institute (DCTI) is a research initiative looking to improve pancreatic beta-cell replacement therapy (islet transplantation) as well as helping improve patient access to such therapies.
Along with other attempts to create islets in the lab (and bypass the problem of donor shortage), these procedures seem to be the future of treatments for type 1 diabetes. On this front, the DCTI is even partnered up with some biotechs, including the well-known Galapagos.
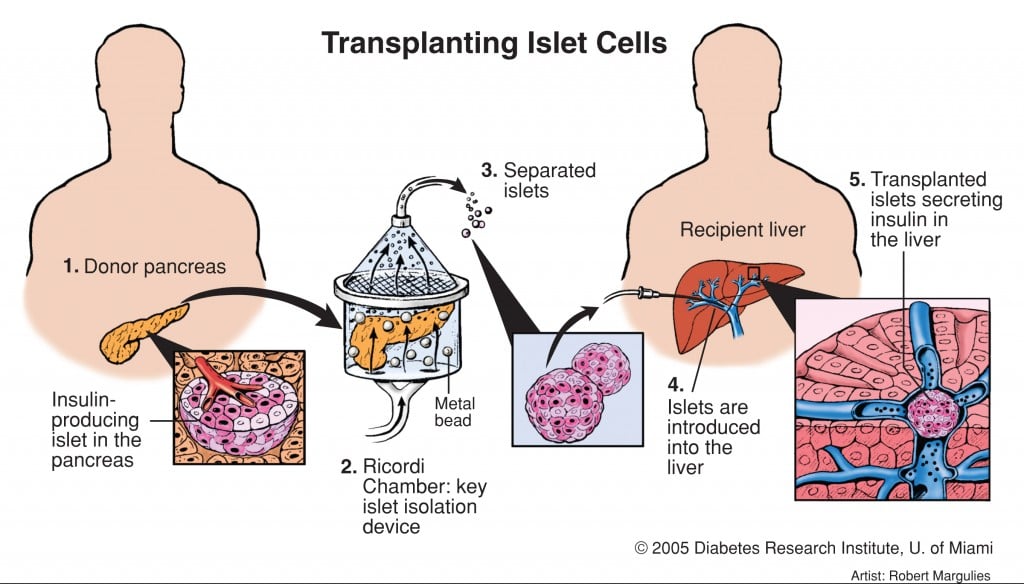
Islet transplants have actually already been around for a while though. In 2000, patients in a trial were able to stop their daily insulin injections after successful transplants – which sounds like the end of diabetes altogether!
But not so fast. The problem was, that although transplantation does technically work, donor islets tend to fail in insulin production over time, which is why the DCTI is looking to optimize pancreatic islet isolation from donors. Therefore, different surgical and transplant methods have been investigated to improve islet transplantation.
Here the DCTI in Groningen (Netherlands) explains the Islet Transplant problem…
Which brings me to news we just covered yesterday – a new surgical method using part of the stomach cell lining has resulted in the successful ‘cure’ of a patient at a hospital in Milan. This is the first example of successful proof-of-concept of this kind of Islet transplant technology in Europe, which was developed at the Diabetes Research Institute (DRI) at the University of Miami.
So is this the end?
So there we have 3 broad areas in which diabetes is being tackled in biotech: new analogs for insulin and insulin-stimulating hormones, improved delivery and monitoring of existing therapies, and lastly, cell therapy.
It is by no means an exhaustive list of all those working in diabetes but will hopefully bring together some of the angles used by the field to approach the disease. Considering that some of these trials are reaping dramatic results, it’s encouraging to keep the scale of how much has been already accomplished in mind.
However, there is much left to still work out, and with patents expiring and many trials still in early stages, it would be overly optimistic to say we’ve made it just yet.
Feature Image Credit: Pancreas and spleen -© Krishnacreations (BioStock ID54709022)
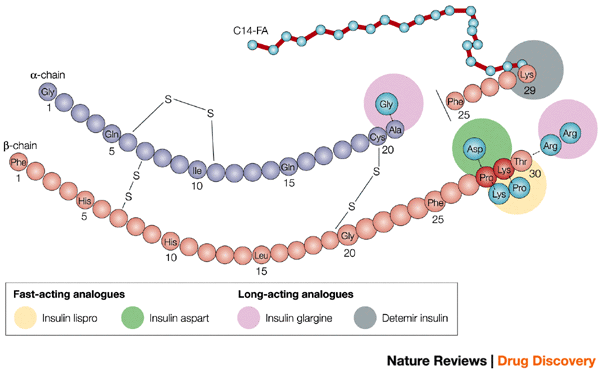
Fig. 1 Owens (2002) New horizons — alternative routes for insulin therapy, Nature Reviews Drug Discovery, 1, 529-540, doi:10.1038/nrd836
Update 17/06/2016: we added mentions to Humulin, made it clearer on delivery issues, Intarcia’s clinical trials,