U.S. gene therapy company Krystal Biotech, Inc, has submitted a Biologics License Application (BLA) to the U.S. Food and Drug Administration (FDA) seeking approval of B-VEC (beremagene geperpavec) for the treatment of patients with dystrophic epidermolysis bullosa (DEB).
B-VEC is an investigational non-invasive, topical gene therapy designed to treat DEB at the molecular level by providing the patient’s skin cells with two copies of the COL7A1 gene to make functional COL7 protein, thereby addressing the fundamental disease-causing mechanism.
“The unmet medical need for DEB patients remains very high and our relentless pursuit of a treatment for this disease continues with the same sense of urgency that we have always had since the founding of Krystal Biotech,” said Suma Krishnan, president of research & development.
Trial data
The BLA submission for B-VEC is supported by data from two placebo controlled clinical trials – the GEM-3 trial and the GEM-1/2 trial.
The GEM-3 trial was designed to evaluate the efficacy and safety of B-VEC for the treatment of DEB. In the trial, matched wounds receiving topical B-VEC or placebo were evaluated in 31 DEB patients over 26 weeks. The pivotal GEM-3 trial met its primary endpoint of complete wound healing at six months and its secondary endpoint of complete wound healing at three months.
The company said B-VEC was well tolerated, with no drug-related serious adverse events or discontinuations due to treatment.
The GEM-1/2 trial looked to evaluate efficacy (mechanistic and clinical) and safety of B-VEC for the treatment of DEB. In the trial, matched wounds receiving topical B-VEC or placebo were evaluated in nine recessive dystrophic epidermolysis bullosa patients over 12 weeks.
Both mechanistic and clinical endpoints were met. No serious or severe B-VEC-related adverse events or systemic drug exposure were noted.
Results from the GEM-1/2 trial of B-VEC for the treatment of DEB were published in Nature Medicine. It showed repeat topical applications of B-VEC were well tolerated and associated with durable wound closure, full-length cutaneous type VII collagen (COL7) expression, and anchoring fibril assembly with minimal reported adverse events.
In addition to submitting the BLA to the FDA, the company is in dialogue with regulatory authorities in other markets, including Europe and Japan. The company anticipates submission of a marketing authorization application with the European Medical Agency (EMA) in the second half of 2022.
Dystrophic Epidermolysis Bullosa
DEB is one of the major forms of a group of conditions called epidermolysis bullosa.
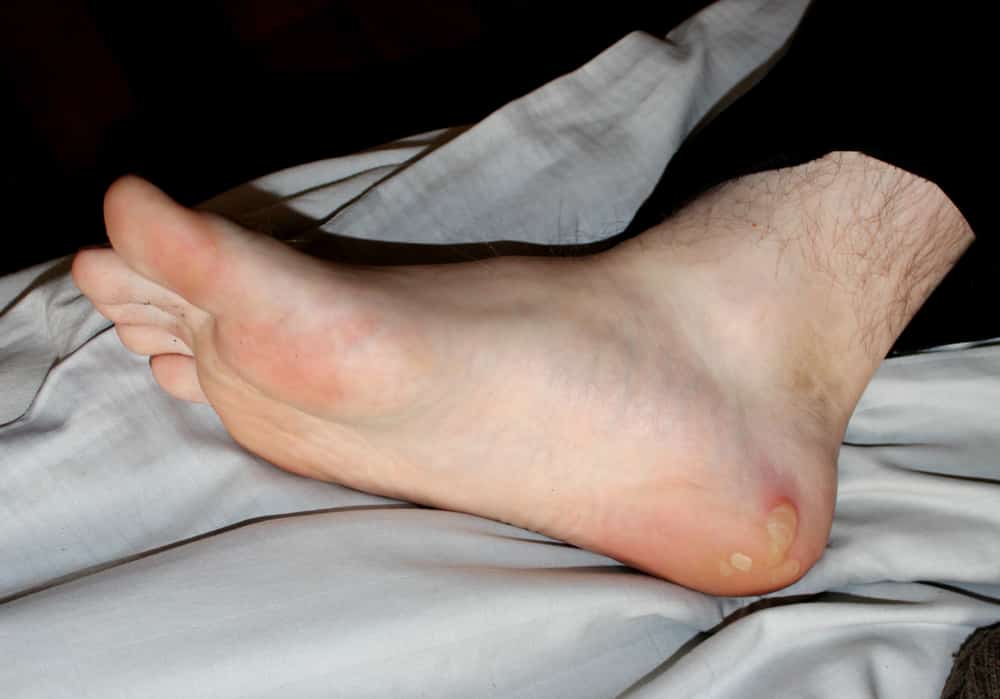
With epidermolysis bullosa, the skin becomes very fragile and blisters easily. Blisters and skin erosions form in response to minor injury or friction, such as rubbing or scratching. The signs and symptoms of dystrophic epidermolysis bullosa vary widely among those affected.
In mild cases, blistering may primarily affect the hands, feet, knees, and elbows. Severe cases involve widespread blistering that can lead to vision loss, scarring, and other serious medical problems.
Dystrophic epidermolysis bullosa is caused by one or more mutations in a gene called COL7A1. This is responsible for the production of the protein type VII collagen (COL7) that forms anchoring fibrils that bind the dermis (inner layer of the skin) to the epidermis (outer layer of the skin).
The lack of functional anchoring fibrils in DEB patients leads to extremely fragile skin that blisters and tears from minor friction or trauma. DEB patients suffer from open wounds, which leads to skin infections, fibrosis which can cause fusion of fingers and toes, and ultimately an increased risk of developing an aggressive form of squamous cell carcinoma which, in severe cases, can be fatal.
Cover image: Shutterstock