Colorectal cancer is the second biggest cancer killer in the US, but Epigenomics could dramatically improve this sad figure.
A problem Berlin-based Epigenomics want to solve and just got a significant milestone: an FDA approval for commercialisation. I met with Thomas Taapken, the CEO of the company, in his office in Berlin, a nice historical building with 4 meters ceiling and close to the city-center.
Epigenomics was started in 1998 from PhDs at the Max Planck Institute for Molecular Genetics here in Berlin. The goal? To build a platform for research on epigenetics, and then commercialize the products. The company IPOed in 2004 on the Frankfurt Stock Market, one of the last German Biotechs to IPO.

By 2006-2007, the company counted 120 employees and 35 research programs. But then the original founders left and instead Epigenomics started on fewer programs, restricted to the diagnostic space only.
Taapken joined in 2011 to restructure the company and bring a clear focus towards the US. He shrank the company to 40 people and instead focused on making this first colorectal blood test work commercially.
Everyone is talking about liquid biopsies now, but we already invented it – 10 years ago.”
So, what is the platform like?
Epigenomics’ platform is, as its name suggests, built on the field of epigenetics. Every tumor releases fragments of DNA fragments like these, called biomarkers, and these sections are very specific.
The Epigenomics platform detects these fragments in order to identify the presence of certain cancers. Epigenomics started with colorectal cancer – as it’s one of the deadliest cancers in the world (see our infographic). It’s the second biggest killer in the US, and in Germany you are 7 times more likely to die from colorectal cancer than in a car crash.
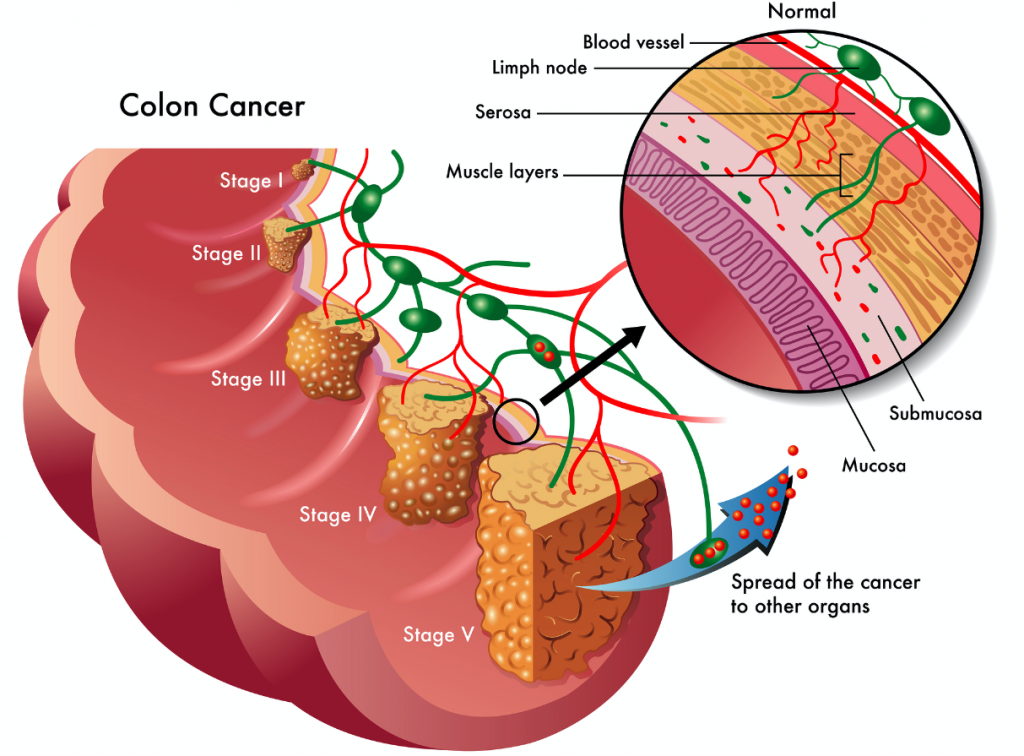
The ‘good’ thing is that “it’s totally preventable if you detect the disease early enough.” In fact, this kind of tumor grows slowly, and if detected in the stages before metastasis, it can be simply removed by surgery. This is the basis for the EpiproColon diagnostic kit.
The company has plans to move toward lung cancer diagnostics as well, where its impact could be even more significant than for colorectal as non-small cell lung cancer is the deadliest.
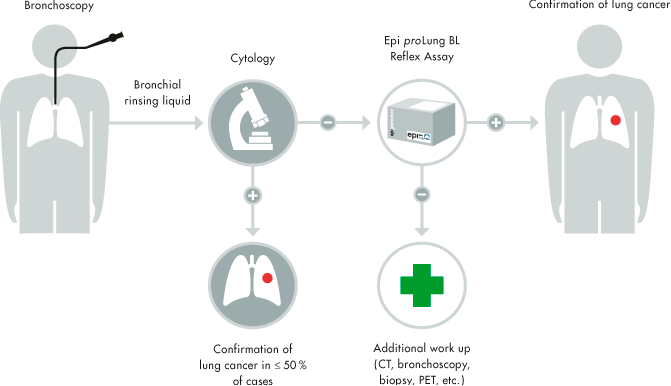
Here’s the challenge: How to make detection of colorectal cancer effective, easily accessible and cheap?
Until now, the standard market test is a stool sample. As the tumor is in the digestive system, it releases blood into the faeces from time to time (which can be screened). This test is cheap and effective – but not convenient.
Eligible people which are offered a screening test often never do it, which is surprising as it seems like minimal trouble for such a big deal as cancer – but people are lazy. Indeed, only 30% of people in France are screened for CRC by a stool test or colonoscopy, and about 40% in Germany actually make use of those methods.
Currently 65% of eligible Americans are getting screened for Colorectal cancer. The American Cancer Society published a goal to get this number up to 80% by 2018. Instead, the advantage of a blood-test is that it can be done at the same time as other blood tests, requiring only an additional 10ml to work. The patient doesn’t need to do anything besides giving blood.

Diagnostics are effective in the fight against cancer
Taapken told me to beat cancer ‘it’s cheaper to develop diagnostics than to cure cancer‘. An early diagnostic test will lead to fewer patients in treatment, aka consuming expensive therapies, and this ultimately results in a reduced cost for the healthcare systems.
And with this in mind, with earlier diagnosis fewer patients are treated too late, meaning fewer patients die. Successful programs have been demonstrated in other cancers (e.g. cervical), and these showed that early diagnoses decreased the number of patient deaths by up to 20-fold.
This FDA approval is a significant milestone for Epigenomics
The test has been sold in Europe (without reimbursement) and China, which accounted for 50% of the companies revenues in 2015. But the FDA and the US market was a key challenge for the company – ‘The market is very different, you have more incentives for such a test’.
Epigenomics started the registration process early 2013 and after a long long long journey, the test has been finally approved! The product will be marketed in partnership with Polymedco in New York, a company well-established in the testing market.
The CEO of Polymedco seems enthusiastic as well:
EpiproColon has the potential to become an important opportunity for laboratories across the country to join the fight on colorectal cancer.”
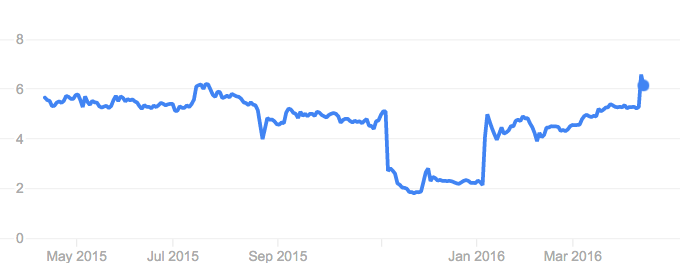
Epigenomics’ stock also increased by 20%
The shareholders and the market seems to be enthusiastic about the good news too. Stock prices increased by up to 20% after the publication of the news. This brings the market cap from €100M to €120M, so to sell at this stage would be a mistake. As Taapken explained, “This approval could pave the way for future successes.”
This FDA approval is great news for Epigenomics, its shareholders and the American patients affected by this terrible disease. I have a close relative who received an early diagnostic test for this cancer, and I can tell you – the earlier the better!
Feature Image Credit: CEO of Epigenomics, Thomas Taapken during Interview with Labiotech (available under CC 3.0 License Labiotech.eu). For a larger version of this image (unedited) drop by our Flickr Account to download and share (all under Creative Commons!).





