Innovent Biologics, Inc. and UNION therapeutics A/S have announced that the first Chinese healthy volunteer has been successfully dosed in the phase I study of orismilast, a potential best-in-class PDE4 inhibitor in global clinical phase II stage.
This study is a multiple dose escalation phase I study in healthy volunteers aiming to evaluate the pharmacokinetic (PK) profile, safety and tolerability of orismilast in healthy Chinese subjects after multiple doses to support the subsequent clinical development of orismilast in multiple indications such as psoriasis and atopic dermatitis (AD).
Orismilast is a next-generation PDE4 inhibitor with high potency for the PDE4 subtypes linked to inflammation. In 2021, Innovent reached a strategic cooperation with UNION to obtain exclusive rights to the research, development and commercialization of orismilast in China and Taiwan.
Orismilast has generated positive proof of concept (PoC) data in psoriasis (administered orally) and in AD (administered topically) and is being developed as a potential best- or first-in-class oral treatment option in both diseases. Compared to other PDE4 inhibitors, the selectivity of orismilast for PDE4 subtypes B and D and the novel modified release delivery is expected to generate a favorable therapeutic window, potentially resulting in improved efficacy and tolerability.
Accelerating development
Lei Qian, vice president of clinical development of Innovent, said: “There is currently no cure for psoriasis, and there is a great unmet clinical need in this field. Orismilast is a new target molecule Innovent laid out in the field of autoimmunity by co-development with UNION. It is potentially one of the best candidates in the mid-stage of clinical development at Innovent. The results of ex-China clinical studies have demonstrated that orismilast has good safety profile and biological activity.
“The ongoing phase I study will evaluate the safety and tolerability of orismilast in Chinese healthy volunteers and provide the basis for further clinical development. We will accelerate the clinical development of orismilast in Chinese subjects with psoriasis or AD in order to obtain regulatory approval as soon as possible in China and to fulfill the needs of safe, effective and convenient long-term oral treatment for patients, thus significantly reducing the disease burden of patients and their pain. We will truly uphold our mission of “To develop and commercialize high-quality biopharmaceuticals that are affordable to ordinary people.”
Kim Kjøller, CEO of UNION therapeutics, added: ‘We are excited by the strong advancement of orismilast by Innovent, which is essential in our joint efforts to provide this novel treatment to patients with dermatological diseases globally. We have seen good progress in our three late-stage clinical trials with orismilast for treatment of psoriasis, atopic dermatitis, and hidradenitis suppurative, respectively. This, together with the now ongoing phase I study in China, is important steps in the development of orismilast.’
About psoriasis and atopic dermatitis (AD)
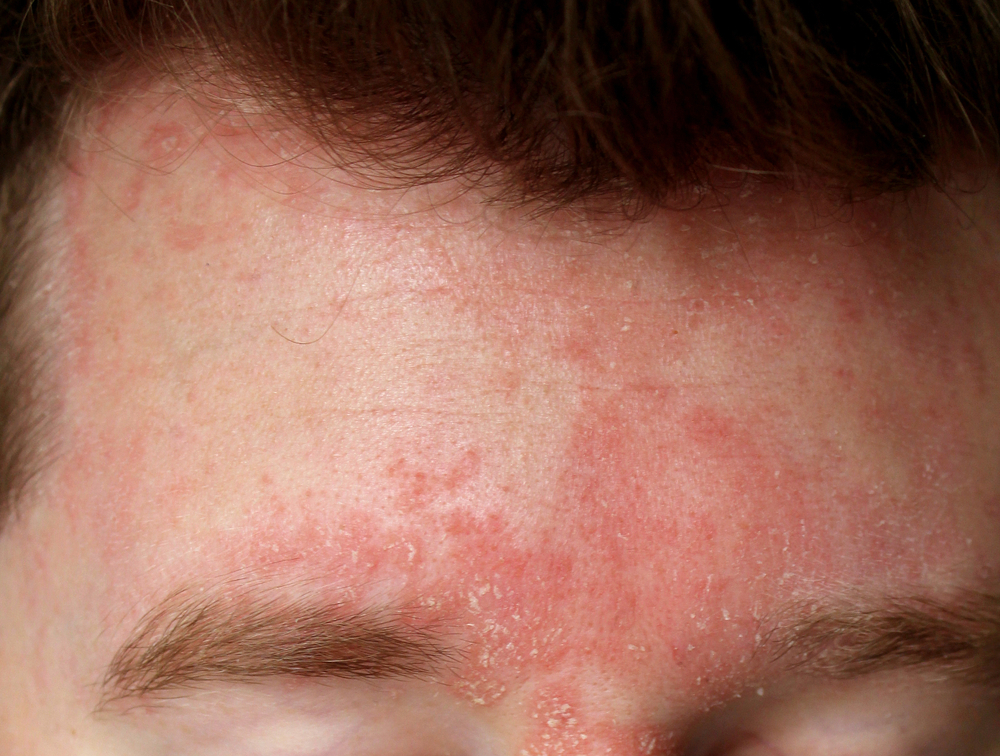
Psoriasis is a multisystem disease characterized by well-defined erythematous scaly plaques that are often pruritic. .Psoriasis is a common disease that occurs more frequently with age. The most common clinical type of psoriasis is psoriasis vulgaris (plaque psoriasis). The involvement of inflammatory cells (e.g., T cells and myeloid dendritic cells) and pro-inflammatory cytokines (e.g., tumor necrosis factor-α, interleukin (IL) -12, and IL-17) in the pathogenesis of psoriasis has been well documented [2].
There is currently no cure for psoriasis. Psoriasis vulgaris can be managed with local therapy, phototherapy, or systemic therapy or any combination thereof.
AD is a chronic inflammatory disease characterized by eczematous lesions and intense pruritus, affecting up to 20% of children and up to 3% of adults.
AD is usually treated with topical therapies, primarily intermittent prophylactic use of topical corticosteroids and/or topical calcineurin inhibitors, along with daily emollients. Intermittent corticosteroid use poses minimal risk of corticosteroid-related adverse effects such as skin atrophy, striae formation in sensitive or thin skin areas, and systemic effects.
About Orismilast
IBI353 (Orismilast) is a next-generation PDE4 inhibitor with high potency for the PDE4 subtypes linked to inflammation currently under development as an oral treatment for psoriasis, AD and HS.
PDE4 is a cyclic adenosine monophosphate (cAMP)-specific PDE that is expressed by immune and inflammatory cells, including T lymphocytes, neutrophils, eosinophils, monocytes, dendritic cells, and macrophages.
In these cells, PDE4 is the predominant PDE form and PDE4 inhibitors increase cAMP levels. High cAMP levels tend to reduce proliferation and cytokine production, whereas low concentrations of cAMP have the opposite effects. PDE4 inhibition has previously been shown to downregulate production of a range of proinflammatory cytokines.
Consistent with previously published work, orismilast demonstrates a potent and broad anti-inflammatory effect by inhibiting the secretion of Th1 (TNFα and IFNg, Th17 (IL-22 and IL-23), and Th2 (IL-4, IL-5 and IL-13) effector cytokines in human PBMCs. In addition, potent inhibition of innate cytokines (IL-1α and IL-1b ) was also observed. These results support further development of orismilast as a potential best-in-class or first-in-class PDE4 inhibitor for the treatment of chronic inflammatory skin diseases including psoriasis, HS and AD.