MaaT Pharma (France) has raised €10M in a series A round, which will allow it to start clinical development of its autologous therapy for imbalance of the Gut Microbiome.
 MaaT Pharma is developing microbiome-based therapies with an autologous (self-sourced) approach. This means that the patient suffering from dysbiosis is treated with their own microbiome, samples of which were stored when the patient was healthy.
MaaT Pharma is developing microbiome-based therapies with an autologous (self-sourced) approach. This means that the patient suffering from dysbiosis is treated with their own microbiome, samples of which were stored when the patient was healthy.
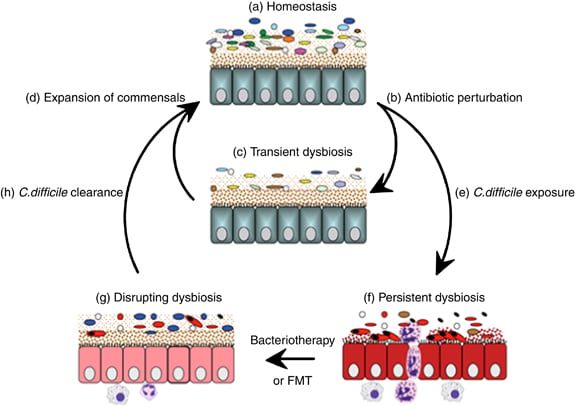
Dysbiosis is an imbalance in the normal microbiota living in the human gut that can lead to difficult opportunitistic infections.
Since its creation in late 2014, MaaT Pharma has attracted significant attention. This Lyon-based startup had already received €2M from the world-first €160M Microbiome Fund, and developed its pre-clinical proof of concept in collaboration with the French National Institute of Agricultural Research (INRA).
MaaT has also won the ‘iiAwards’ startup competition and was selected for the TOP10 in the Big Booster programme.
MaaT Pharma has now raised an additional €10M, from its previous investors (Seventure and INRA Transfert) and two new investors, the CM-CIC (who also invested in other cool French startups) and Biocodex (France), a pharma that is already developing probiotics and therapies in gastroenterology.
The new funds will help MaaT enter clinical trials with its current projects. These include the therapy itself (MaaT001), which is a type of fecal microbiota transplantation (FMT), as well as a prototype to safely collect and store stool samples (MaaT002).

For CM-CIC (one of the new investors), a key factor for its interest in MaaT was that they combined an autologous therapy with an an industrial, quantifiable and reproducible process – a challenge for the industry.
While autologous therapies carry less risks of medical complications (like immune rejection), these strategies face significant challenges in manufacturing and logistics – something that has been discussed a lot in the CAR-T field, for example, with great arguments supporting universal approaches.
Upcoming clinical trials will study patients that developed dysbiosis after treatments (that can hurt the microbiome) for leukemia or bone and joint infections. So, collection of microbiome samples would happen before these treatments were carried out.
MaaT Pharma seems to have its autologous strategy well thought-out, which has therefore granted them important financial backing. This startup could really become an important player in the emerging Microbiome field.





