Genticel is a step closer to prevent cancer caused by the human papillomavirus (HPV). The French biotech announced positive results of GTL002’s preclinical in vivo proof of concept, Genticel’s multivalent HPV therapeutic vaccine candidate based on the company’s versatile Vaxiclase platform.
Vaxiclase is a technology platform that can be used with many antigens in several indications. Last February, Genticel signed a first licensing agreement with the Serum Institute of India, the world’s largest producer of vaccine doses, to evaluate Vaxiclase to produce multivalent vaccines against Pertussis, the whooping cough disease. Genticel received up to €51M in upfront and milestones payments, plus royalties on sales.
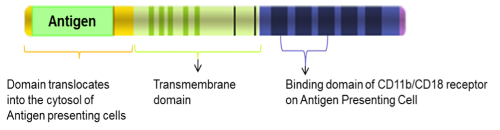
The company is spending the cash well, developing its new candidate, GTL002. The proof of concept data of the drug demonstrated that an in vivo immune response was induced against each of the six HPV-derived proteins present in the therapeutic vaccine. In addition, in vivo therapeutic efficacy has been proven after tumor eradication in the most widely used and broadly accepted reference model.
No treatment options are available for women infected with oncogenic HPV types who have not yet developed high-grade lesions or cervical cancer. Genticel’s GTL002 seeks to eradicate the six most oncogenic HPV types affecting 158 million women worldwide.
With its two leading molecules, Genticel is the first company to have established a staged pipeline of HPV therapeutic vaccines for the large female population burdened with this unmet medical need. The company also owns GTL001, known in Europe as ProCervix, a first-in-class therapeutic vaccine candidate currently in phase II in Europe that already targets the two most oncogenic HPV types that affect 93 million women.





