Cologne based Cevec specialises in producing tailor-made recombinant glycoproteins and gene therapy vectors. Now it has announced a breakthrough in their adenoviral vectors used in Gene Therapy.
 The increasing number of gene therapy-based discovery programs in the biotech and pharma industry is driving a greater need for the scalable production of gene therapy vectors.
The increasing number of gene therapy-based discovery programs in the biotech and pharma industry is driving a greater need for the scalable production of gene therapy vectors.
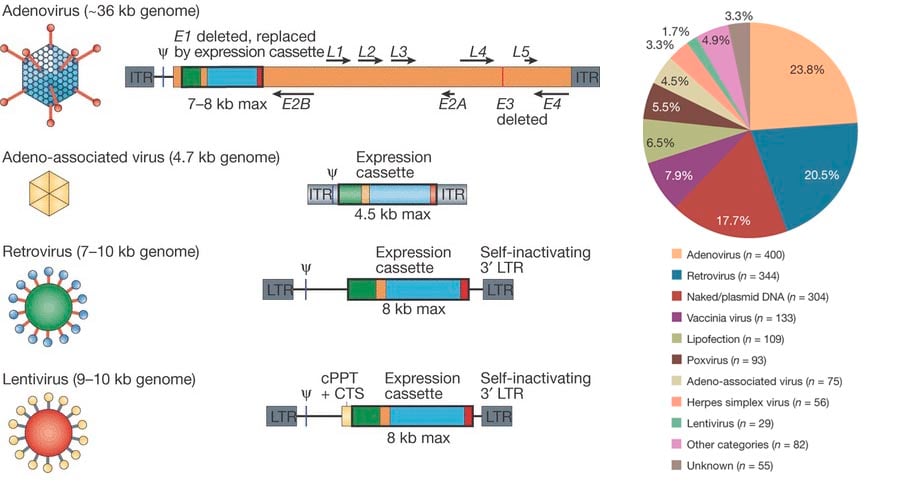
Vectors are needed to transport a desired gene to the patient, and simple viruses are a popular choice to do so.
Examples of biotechs which have trials using Adeno-assosciated viral vectors include Lysogene (working on the rare disease San Filippo syndrome with adeno-associated viral vectors), GenSight (looking to ‘cure blindness’ using adenoviral vectors) and Theravectys (using lentiviral vectors for vaccines against Leukemia).
However, the problem is production of these vectors to comply with GMP standards and to ensure their safety (as viral vectors) by rendering them incapable of self-replication, to prevent them from transfecting cells outside the targeted tissue area.
Now Cevec’s proprietary cell-expression system (CAP GT) has delivered the production of safe adenoviral (AV) gene therapy vectors with the proven absence of replication-competent adenovirus (RCAs).
For most adenoviral-based gene therapy applications, adenoviral vectors are modified in such a way to deliver the gene of interest to the target cell, but cannot replicate within the cells of the patient.
The commonly used producer cell line for vector production, HEK 293, bears the inherent risk of accidentally generating replication-competent adenoviruses (by homologous recombination).
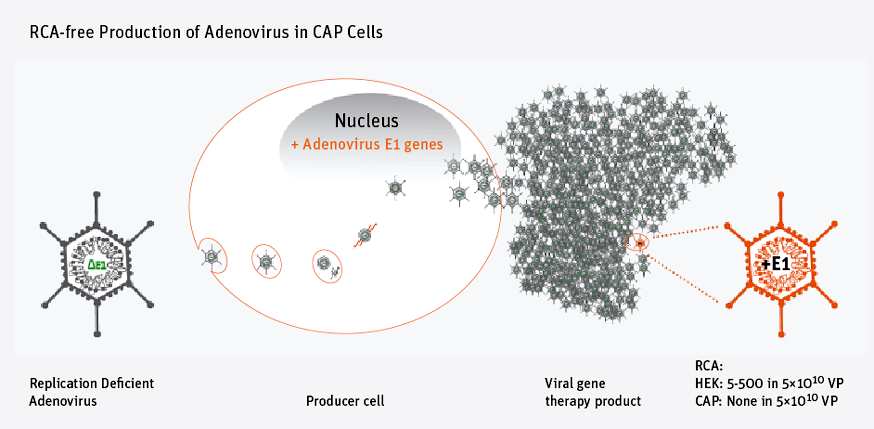
The absence of RCAs in clinical production lots can only be proven through intensive testing in order to eliminate the risk of involuntarily spreading the infective vector.
But Cevec’s CAP GT cell has now been demonstrated to prohibit the formation of RCAs, as the CAP cells do not allow homologous recombination events to occur with the AV vector genome.
This is a great demonstration of Cevec’s technology, and the company is preparing to submit work to the FDA on their cell lines.





