Despite progress in HIV treatments and towards an HIV cure, the infection rate has risen to unprecedented levels in Europe. Gilead’s HIV-1 ‘vaccine’, Truvada, has just been approved for marketing in Europe to stop its spread.
 The year 2014 brought the highest number of new HIV diagnoses ever recorded in Europe, 94% of which were a result of sexual transmission. Gilead has been attacking the disease from all angles: it has been working on an HIV cure with Genmab and first developed Truvada for basic disease management. It entered the European market in 2005 for this purpose; now, the company has attained approval to expand Truvada’s use and market it to high-risk individuals not yet infected with HIV-1 as a prophylaxis.
The year 2014 brought the highest number of new HIV diagnoses ever recorded in Europe, 94% of which were a result of sexual transmission. Gilead has been attacking the disease from all angles: it has been working on an HIV cure with Genmab and first developed Truvada for basic disease management. It entered the European market in 2005 for this purpose; now, the company has attained approval to expand Truvada’s use and market it to high-risk individuals not yet infected with HIV-1 as a prophylaxis.
The European Commission has greenlighted the use of Truvada in a preventative strategy, known as Pre-Exposure Prophylaxis (PrEP), for people in danger of contracting HIV-1. The EU now joins Australia, Canada, Kenya, Peru, South Africa and the US in the club of countries who have approved Truvada for PrEP. Of course, the drug will still be subject to monitoring, regulation, and approval by individual nations in the Union, and it must clear these hurdles before it becomes ubiquitous on the continent.

Truvada is an antiretroviral medication that until now was prescribed in a combination therapy to manage HIV-1 in people afflicted with the disease. It is a mixture of emtricitabine and tenofovir disoproxil, small molecules resembling parts of DNA that suppress virus replication. Truvada is typically one component of a three-part cocktail formulated to minimise the drug resistance; it is not a complete regimen, as HIV-1 resistance has emerged in individuals taking it alone.
Since individual European countries will have regulatory control over Truvada 2.0, pricing is yet to be determined. However, these treatments are generally expensive, and Gilead is not known for being generous to consumers when valuing its drugs. Gilead also emphasises that this incarnation of Truvada remains part of a holistic regimen of safe sex practices like condom use to prevent the spread of HIV.
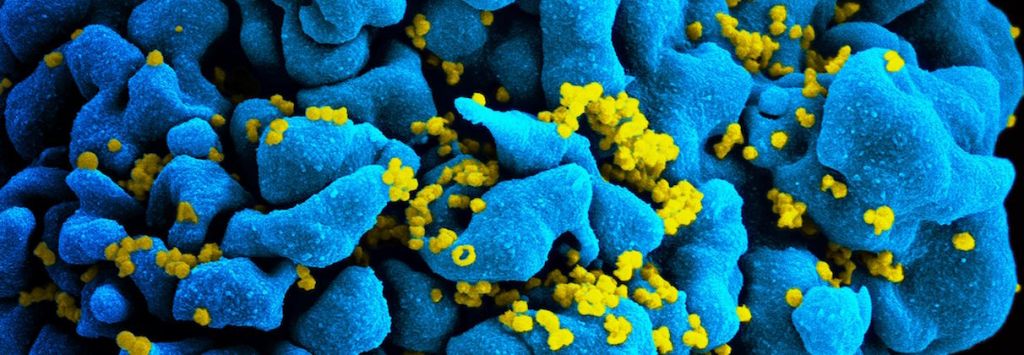
Featured Image: HIV-infected T cell (CC2.0, NIAID/Flickr)
Figure 1: Grab a Condom Hoop Art (CC2.0, Hey Paul Studios/Flickr)