The second-ever gene therapy to get European approval finally has a price tag. It will be sold at ‘only’ €594k, but with a twist – it has a money-back guarantee. Could this strategy finally get gene therapies some sales action?
 In May, GSK finally got European approval for a gene therapy, after sparking the resurgence of the field. Named Strimvelis, the therapy is a one-time cure for a very rare type of severe combined immunodeficiency (SCID) due to adenosine deaminase deficiency (ADA-SCID).
In May, GSK finally got European approval for a gene therapy, after sparking the resurgence of the field. Named Strimvelis, the therapy is a one-time cure for a very rare type of severe combined immunodeficiency (SCID) due to adenosine deaminase deficiency (ADA-SCID).
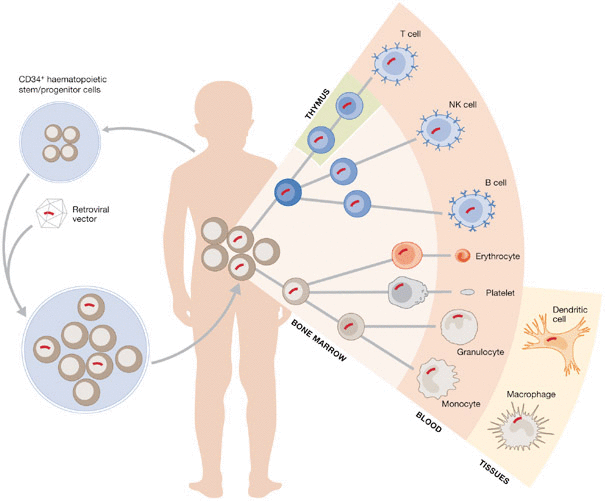
Strimvelis consists of an ex vivo procedure, where a copy of the enzyme’s gene is inserted into the bone marrow stem cells of patients. This treatment offers an alternative to bone marrow transplants, which require suitable donors, and enzyme replacement therapy, which requires lifelong regular injections.
Now, the price tag of Strimvelis is finally set at €594k, after negotiations with the Italian Medicines Agency (AIFA). As the therapy will only be administered at the Ospedale San Raffaele in Milan, this becomes its effective price.
However, the most interesting twist may be that GSK has included what is basically a money-back guarantee scheme: should the gene therapy not succeed in curing a patient, GSK will return his or her money. Additionally, the treatment can be paid in installments over a period of years.
Strimvelis’ price is nearly half of that of Glybera, which broke the €1M record as the first-ever gene therapy to be approved in a Western country. With such a high price, Glybera proved difficult to prescribe and was a commercial flop.
GSK had already hinted that it would target cheaper gene therapies even if it meant eating the costs, something not so well-received by the industry (see the gene therapy panel at Refresh).
Still, the MIT Technology Review says the current price was the result of tough bargaining by the AIFA. Strimvelis is now quite competitive, as bone marrow transplants can cost as much as $1M and enzyme replacement therapy is around $250k a year.
The refund strategy is far from common for a Pharma product. However, such adaptations are now on the table as possible solutions to the difficult commercialization of gene therapies. Strimvelis’ sales model could set the standard for the future of the field.
Images: GSK; Sauer et al. (2012) Frontiers in Immunology (doi: 10.3389/fimmu.2012.00265); Mavilio and Ferrari (2008) EMBO Reports (doi: 10.1038/embor.2008.81)





