A new study published in the journal Science Translational Medicine identifies a new treatment strategy to prevent GVHD after stem cell transplantation.
Allogeneic stem cell transplantation remains one of the top treatments for blood cancers and inherited blood disorders, but the treatment is associated with a major risk of so-called graft-versus-host disease (GVHD). A new paper published by an international team of scientists from the Technical University Munich in Germany and the Memorial Sloan-Kettering Cancer Center in the US now describes how this deadly condition could be prevented.
During a stem cell transplant, the hematopoietic compartment residing in the bone marrow of the patient is first destroyed to make room for the new, healthy donor cells. However, about 50% of the patients, experience GVHD after transplantation. Here, the immune cells arising from the stem cell transplant start attacking the patient’s already drained body, instead of going for intruders such as bacteria.
The reported treatment strategy is based on triggering regenerative responses within the gut, where drug treatment and radiation usually cause damage to the epithelial cells of the intestinal mucosa. Such damage to the epithelium leads to the release of stress signals and the arrival of intestinal bacteria in previously germ-free areas of the gut, thereby activating aggressive donor T cells.
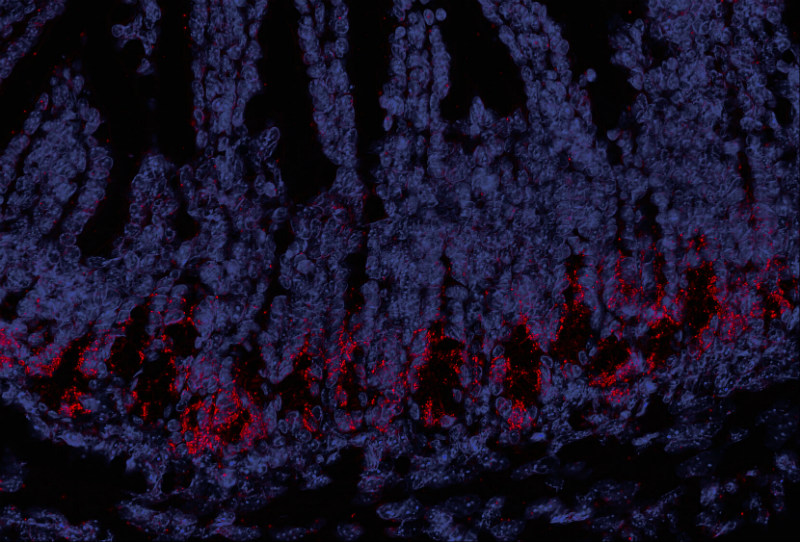
To quickly restore the gut epithelium, the scientists made use of two proteins that are produced naturally in the body during an initial immune response against bacteria and viruses: RIG-I and STING. These molecules usually activate signaling pathways that trigger immune responses, but the scientists could also show that the molecules can also bring about a regenerative effect.
When the scientists stimulated the RIG-I pathway using triphosphate-RNA, the natural ligand of the RIG-I receptor, they could demonstrate a protective effect on the gut epithelium, which in mouse models also lead to a reduction of GVHD after allogeneic hematopoietic stem cell transplantation.
“Both RIG-I agonists, such as 3pRNA, and STING agonists are currently in clinical development,” Hendrik Poeck, co-head of the study, said in a press release from the TUM. “It thus appears quite possible that these selective agonists will be administered in the future to patients who are candidates for allogeneic stem cell transplants. However, further studies will be needed to learn how they actually work before applications in human medicine are possible.”
Indeed, the successful Munich-based biotech Rigontec is currently developing such a RIG-I agonist, based on its promising anti-tumor effects. The current study now points to an even wider range of potential applications of this exciting new drug candidate.
Images via shutterstock.com / nobeastsofierce and mediatum.ub.tum.de