Heptares Therapeutics has published the detailed structure and mechanism of action of a difficult-to-target GPCR protein with great therapeutic prospects.
Heptares Therapeutics has published today in Nature new insights into the mechanism of action of antagonists of the protease-activated receptor 2 (PAR2), a molecule involved in metabolism and inflammatory responses. The study is part of a collaboration between Heptares and AstraZeneca in which both teams apply their unique expertise to discover and identify novel drugs against historically undruggable G protein-coupled receptors (GPCRs)
Although well-validated target for multiple indications in the areas of pain, cancer and inflammatory disease, PAR2 could not be used for drug discovery using conventional methods. The reason is that the protein’s activation mechanism is unusual for a GPCR; it requires a cleaved part of the own receptor to act as a ligand.
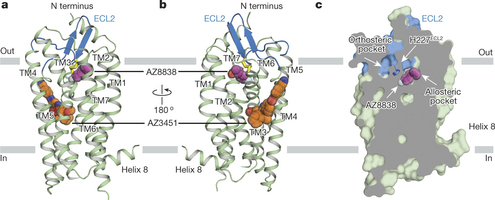
The study describes how two molecules identified by AstraZeneca bind PAR2. The first, AZ8838, binds the GPCR protein in a previously inaccessible pocket near the extracellular region, whereas AZ3451 can bind a remote allosteric site that can prevent the activation of the receptor.
The news follows the recent announcement of a €11M milestone payment that Heptares Therapeutics received from AstraZeneca after the checkpoint inhibitor AZD4635 successfully completed preclinical trials. The team seems clearly prepared to tackle the difficult task of turning difficult-to-drug targets into new approaches to treating disease. However, they will have to look out for possible competitors such as the young Confo Therapeutics, a Belgian biotech seeking to outperform the current standard of GPCR technology.
Images from Bakhtiar Zein /Shutterstock; RKY Cheng et al. Nature (2017)





